![]()
Imaging in Cerebrovascular Disease | 1 |
Christopher P. Hess
Department of Radiology and Biomedical Imaging,
University of California, San Francisco,
San Francisco, California, USA
The past two decades have seen tremendous advances in our understanding of the anatomy and physiology of the central nervous system and its supporting vasculature. The lesion studies, anatomic dissection, and light microscopy techniques of the past have been largely supplanted over the last three decades by modern neuroimaging, which now uniquely permits non-invasive and in vivo visualization of the brain’s structure and physiology in the individual patient. These tools serve as the cornerstone for the detection, characterization, and monitoring of all cerebrovascular abnormalities, for open surgical, endovascular and radiosurgical treatment planning, and for follow-up after clinical management. Imaging is also essential for patient selection in clinical trials and, in some cases, can serve as a powerful marker to predict patient outcomes.
Cerebrovascular imaging has evolved at a rapid pace, to a point where there are now multiple modalities in routine use, each with its own dizzying array of specialized techniques that characterize specific anatomic or physiologic features of disease. Computed tomography (CT), magnetic resonance imaging (MRI), and digital subtraction catheter angiography (DSA) serve as the three core imaging modalities for vascular imaging in current practice. Each provides a complementary lens through which to view the biological complexity of cerebrovascular disease; in some cases, more than one modality is necessary.
This chapter reviews the current state of the art in cerebrovascular imaging and describes the fundamental strengths and limitations of the three key modalities. The primary goal is to provide the reader with a general, “30,000-foot” overview of the techniques used in current clinical practice to image vessels, blood flow and the brain’s microcirculation. The first section discusses the original and most commonly employed imaging tool to evaluate the intracranial vascular system: angiographic imaging of the vessel lumen. The sections that follow outline techniques for dynamic (time-resolved) angiography, and methods for quantifying brain physiology from the microcirculation. The final section describes the importance of brain parenchymal imaging for the evaluation of cerebrovascular disease.
1.1Imaging of the Arterial and Venous Lumen
The most frequently used methods for imaging of cerebrovascular system are based upon visualization of blood within the vascular lumen using X-ray, CT, or MR angiography.
1.1.1CT angiography (CTA/CTV)
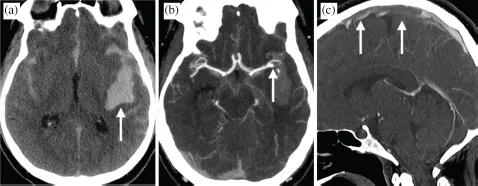
Because differences in the intrinsic density of the blood pool and the vessel wall are visually imperceptible, luminal contrast is generated with CT via bolus injection of dense iodinated contrast and rapid imaging of the head during transit of the contrast bolus through the cerebral circulation. Images are acquired as intravascular contrast medium extends into patent and opacified cerebral vessels. Modern multidetector CT (MDCT) enables coverage of the entire head and neck within 4–8 seconds, and first-pass transit for the contrast bonus from the arterial to the venous circulation takes place in less than 10 seconds. By deliberately timing the acquisition of CT images with respect to the anticipated location of the contrast bolus within the circulation, images can be obtained to emphasize the arterial and/or venous system (Figure 1). CT is also most often performed prior to any contrast injection in order to identify the presence of acute hemorrhage and other structural abnormalities that might require immediate patient care, for a full exam time of less than 5 minutes.
The high density of injected iodinated contrast relative to other brain and neck soft tissues allows CT to reveal the patent vascular lumen within the cervical and intracranial circulation with very high spatial resolution (typical MDCT voxel sizes are 0.5–1 mm). This resolution equips CT arteriography (CTA) with the ability to characterize stenotic disease with extremely high accuracy, to identify contour irregularities and aneurysms in arteries of the circle of Willis and cortical branches, and to detect arterial occlusions in major vessels. To better study the venous system, high-resolution CT venography (CTV) is performed in an analogous fashion to CTA, but with delayed timing of MDCT acquisition relative to the bolus injection (usually around 45 seconds).
An important limitation of CTA arises in the setting of occlusive or severe stenotic arterial disease. In these low-flow situations, the injected contrast bolus may not opacify the lumen of arteries upstream of the site of disease during the time of CT acquisition. This limitation becomes particularly important in the setting of arterial thrombus in stroke, as it precludes accurate determination of the length of the thrombus (an important consideration for planning endovascular treatment). To overcome this shortfall, many centers repeat CT at a later venous phase, with sufficient delay to permit contrast to extend into the vessel of interest if it remains patent.
Given its ability to rapidly acquire images, widespread availability in hospitals and emergency departments, and relatively lower cost compared to other modalities, CT/CTA is the most regularly used technique in patients with emergent neurologic disorders. Most neurosurgical emergencies, including large vessel occlusions (LVOs), ruptured aneurysms, hydrocephalus, herniation, and acute hemorrhage, are readily diagnosed using a combination of CT and CTA and/or CTV. However, although vascular contrast is high with bolus CTA, CT suffers from poor soft tissue contrast that can make subtle lesions in the brain parenchyma difficult or impossible to detect. Evaluation of vessels around the bone may also be limited by beam-hardening artifacts. The sensitivity of CT is thus limited for many other disorders. Furthermore, CT/CTA requires radiation for imaging, at a time when medical radiation to patients is gaining attention in the scientific literature and even in the mainstream media (Brenner & Hall, 2007; Smith-Bindman et al., 2009).
1.1.2MR angiography (MRA/MRV)
Like CT, MR angiography can be achieved using bolus injection of contrast media (in the case of MRI, typically a gadolinium chelate) for contrast-enhanced MRA (CE-MRA). Because acquisition of high-resolution MR images requires more time than CT, however, more careful synchronization is required between the acquisition of imaging data and the temporal passage of the bolus through the craniocervical circulation. Optimal timing for acquisition during peak arterial or venous enhancement (for MRA or MRV, respectively) is typically estimated empirically from a separate timing or “tracking” bolus experiment before angiographic imaging to assess first-pass contrast kinetics. Elliptical centric MR acquisition is then used during a second bolus injection to generate luminal images with optimal arterial or venous contrast. With this technique, the second MR acquisition is modified to acquire data containing contrast information early in the scan, during the peak arterial or venous phase of the second administered contrast bolus. Data containing information about edges in the image are acquired later during the scan, a process that takes approximately 2 minutes in total. Using bolus timing and elliptic-centric acquisition, it is possible to perform CE-MRA with very high spatial resolution, usually 0.5 mm in plane with 1–2 mm slice thickness using parallel imaging at 3T. Although this spatial resolution falls short of what can be achieved with CTA, it is more than sufficient to monitor changes in coiled cerebral aneurysms stents (Wallace et al., 2007), without the downside of repeated radiation exposure and artifacts that come with serial CTA.
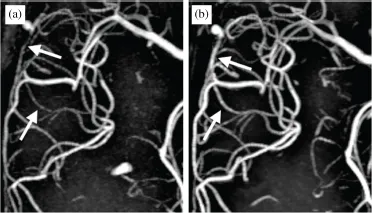
Using time of flight (TOF) MR angiography, it is also possible to obtain excellent images of the vascular lumen in the intracranial circulation without the requirement for intravenous contrast. The ability to study vessels without contrast using TOF MRA and MRV makes these the dominant techniques used in practice for routine screening of patients with suspected arterial or venous disease. Both exploit the principle of flow-related enhancement, in which the MRI signal from stationary tissue is suppressed and that within the vascular lumen is enhanced by sensitizing the signal to flowing blood. The contrast and spatial resolution of TOF MRA and MRV can be further enhanced by using higher field strength, 3T and even 7T, MRI (Figure 2). It is important to note, however, that this method for generating luminal contrast is fundamentally limited when blood flow is slow or turbulent. TOF techniques suffer from significant limitations in this setting, including over-estimation of the degree of narrowing in stenotic disease and failure to accurately depict luminal diameter in complex flow, such as within the carotid bifurcation, giant intracranial aneurysms or arteriovenous malformations (AVMs). In these cases, gadolinium-enhanced CE-MRA is preferred.
In addition to its angiographic capabilities, MRI has higher soft tissue contrast than any other modality, and thus provides the best possible evaluation of the brain parenchyma. In contrast to CT and DSA, it also requires no ionizing radiation. However, MRI requires longer imaging times than CT, often 15–30 minutes for optimal diagnostic quality, which may delay treatment. MRI scanners are also less accessible than CT scanners, and examinations are more costly. Furthermore, patients with severe claustrophobia cannot safely undergo MRI without anesthesia, and MRI is contraindicated in patients with certain implanted devices.
1.1.3Catheter-based digital subtraction angiography
The use of catheter angiography has gradually declined over the last three decades, as the accuracy of non-invasive CT and MRI has improved significantly. Nonetheless, given its inherently high spatial and temporal resolution, it remains the gold standard for evaluation in many vascular disorders. DSA, which differs from the old “cut film” angiography techniques of the past in its temporal subtraction of images to remove bony structures and depict only intravascular contrast, now serves as the de facto standard to evaluate lesion angioarchitecture and understand the dynamics of arterial blood supply to and venous drainage from complicated lesions. DSA also uniquely permits endovascular treatment, including the removal of acute occlusive thrombi in stroke and transcatheter treatment of aneurysms, fistulas, and tumor blood supply.
There are a number of disadvantages to DSA. It is an invasive technique that requires direct arterial or venous cannulation, catheter and wire manipulation for vessel selection, and injection of relatively high volumes of contrast media. Large post-procedural groin hematomas, internal bleeding related to vessel puncture, and significant vascular injury requiring surgical repair are extremely rare in experienced hands. However, even with the best technique, there is a small but finite risk of causing transient ischemic attack, stroke, or extremity ischemia when thrombus forms at the tip of the catheter, plaque is dislodged from aortic or other arterial atheroma, or small volumes of air are inadvertently injected. According to a cooperative statement issued by the Society of Interventional Radiology, the American Society of Interventional and Therapeutic Neuroradiology, and the American Society of Neuroradiology, the reported range of reversible neurological deficits with DSA ranges from 0 to 2.3%, and that of permanent neurological deficit, 0 to 5% (Citron et al., 2003). A second drawback of DSA is its requirement for significantly higher doses of radiation than CTA in most cases. Although dose can be reduced using high-sensitivity detectors, image noise reduction techniques, and low frame-rate acquisition, recent estimates for effective radiation dose are 2–7 times higher for cranial DSA than CTA, averaging 2.71 mSv for DSA (by comparison, averaging 0.67 mSv for CTA) (Manninen et al., 2012). Effective DSA doses are even higher in patients undergoing catheter-directed interventions.
Other disadvantages of DSA are its requirement for relatively larger contrast volumes and the inherent limitations that come with planar radiography. The former is especially problematic in patients with diminished renal function at baseline, as large-volume injected arterial contrast may ...