![]()
1. Introduction
Carbohydrates are naturally present in plants (70–75% of dry matter) and animals (5–10% of dry matter). They are mainly formed in green plants in a multitude of biosynthetic pathways from the products of photosynthetic reactions; their structures range from various monomers to giant polymer molecules. Carbohydrates serve as basic energy sources (sugars) or reserve fuels (starch and glycogen), but they also form supporting structures in plants and certain animals (cellulose and chitin). Carbohydrates and their derivatives are also found as structural members in numerous biologically important substances, such as nucleic acids (DNA and RNA).
The name “carbohydrate” was initially connected with compounds that could be seen as various hydrates of carbon and whose elemental composition followed the experimentally found formula Cn(H2O)m (n > 2). Common compounds with a sweet taste, such as xylose C5(H2O)5, glucose C6(H2O)6, and sucrose C12(H2O)11, were typically counted as carbohydrates. Nowadays, we know many carbohydrates — for example, deoxyribose C5H10O4 — that do not follow this definition. On the other hand, enormous numbers of compounds exist that could be considered carbohydrates based on their elemental composition, but their chemical behavior is completely different. This shows that the traditional definition of carbohydrates is too narrow as the only criterion; the name is also misleading because they do not contain water molecules in their structures. In a broader context, these compounds can instead be seen as polyhydroxy compounds (R-(OH)n) that either contain, or whose acid hydrolysis products contain, an aldehyde group (R-CHO, in aldoses) or a ketone group (R-CO-R′ in ketoses).
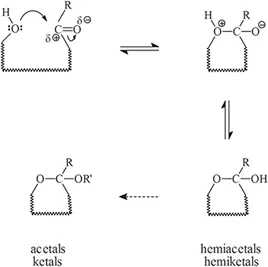
In practice, carbohydrates are mostly present as various ring structures where the functional hydroxyl and carbonyl groups participate in the formation of these cyclic structures. Among such structures are aldehyde-based hemiacetals (cyclic hemiacetals) and their derivatives, acetals, and the corresponding ketone-based hemiketals (cyclic hemiketals), and their derivatives, ketals. In aldoses, R is H and in ketoses, R is generally CH2OH; the so-called “aglycone” part R’ is normally an alkyl group — the carbohydrate part is then called a “glycone”:
It is rather difficult to give a simple characterization of the carbohydrate group of compounds because the traditional definition mentioned above only applies to “basic” carbohydrates but not to their many derivatives. Carbohydrates found in nature often contain other functional groups, for example, in compounds, such as sugar phosphates, sugar sulfates, amino sugars, and thiosugars. In addition, there are cases, such as well-known sugar alcohols (e.g., xylitol, mannitol, and glucitol or sorbitol), where the carbonyl group has been reduced to the corresponding alcohol. The formed polyhydroxy-alkanes have properties similar to carbohydrates, and they are commonly considered as carbohydrate derivatives. Similarly, polyhydroxycycloalkanes (e.g., inositols) resemble carbohydrates and are thus seen to belong to them.
There are also numerous pectins in nature, such as polyuronides, consisting of oxidized monosaccharide units, uronic acids. Because of the importance of carbohydrates in a variety of biochemical reactions, they are structural constituents, besides in the nucleic acids mentioned above, also in glycoproteins and glycolipids (so-called “glycoconjugates”). These conjugate molecules are no longer deemed pure carbohydrate derivatives; their classification among the compound groups of organic chemistry is primarily based on the parts of the molecule other than the carbohydrate component.
Carbohydrates are divided into three subgroups on the basis of their structure: (i) monosaccharides, (ii) oligosaccharides, and (iii) polysaccharides (as a group generally called “glycans”). These prefixes come from Greek, where “mono” means “one”, “oligo” “a few”, and “poly” “many”. Monosaccharides are simple sugars, like ribose, xylose, glucose, and mannose, which cannot be hydrolyzed into smaller “carbohydrate units”. Oligosaccharides comprise a few (two to nine) monosaccharide units linked together with glycosidic bonds formed by splitting water molecules. They are divided according to their number of monosaccharide units into disaccharides, such as lactose, maltose, sucrose, and cellobiose, trisaccharides, such as raffinose, and tetrasaccharides, such as stachyose, and so on. Except disaccharides, only a few oligosaccharides are found in nature. On the contrary, an abundance of natural polysaccharides are found: cellulose, starch, and hemicelluloses, such as xylans and glucomannans, contain monosaccharide units (their number can vary from ten to thousands) linked together with glycosidic bonds. In acid hydrolysis, the oligo- and polysaccharides can yield the same or dissimilar monosaccharides. If all these monomeric building blocks are identical, we speak about homopolysaccharides, such as cellulose and starch; otherwise we are dealing with heteropolysaccharides, such as hemicelluloses.
It is also possible to classify carbohydrates according to their solubility in water. Besides readily water-soluble carbohydrates (sugars), there are polymeric carbohydrates that only swell in water and form colloidal solutions (e.g., starches). Cellulose and other related polysaccharides are insoluble in water. Sugars are sweet and often exist as crystals, although they can also form syrups that are difficult to crystallize. Because of their many intermolecular hydrogen bonds, sugar crystals have generally a higher density and strength than the crystals of other organic compounds. This is also why sugars do not markedly dissolve in solvents, such as diethyl ether, chloroform, or toluene, which lack hydroxyl groups needed to form hydrogen bonds.
Carbohydrate structures often contain numerous asymmetric carbon atoms (called “chiral centers”) whose four substituents are all different. Because of these chiral centers, such carbohydrates are often optically active and rotate the plane of plane-polarized (linearly polarized) light either to the right ((+)-form) or to the left ((−)-form). This optical activity arises from the three-dimensional structure of the compound and leads to the central importance of stereochemistry, a branch of organic chemistry that examines, for example, the spatial structure of carbohydrates. It can be claimed that a complete understanding of carbohydrate chemistry is not possible without a profound knowledge of the stereochemistry of either the simple molecules or the macromolecules examined.
In the context of certain carbohydrates, the term “reducing sugars” is used; this refers to the ability of their free aldehyde group (being present as hemiacetal) to reduce, for example, Fehling’s solution. This property has been traditionally used for non-specific detection of sugars. In certain cases, such as sucrose which belongs to disaccharides and consists of two monosaccharide moieties, glucose and fructose, the hydroxyl group in the hemiacetal structure of glucose (an anomeric hydroxyl group) and the corresponding hydroxyl group in fructose’s hemiketal structure form a mutual glycosidic bond. In this case, the above reduction process is no longer possible; hence, this carbohydrate belongs to “non-reducing sugars”. The reducing end group in polysaccharides is of great importance in the carbohydrate degradation reactions that reduce the total cooking yield in alkaline pulping (cf., Chapt. “11.2. Polysaccharides”).
Many small-molecule carbohydrates (the most common mono- and disaccharides) are widely used, among others, in the food and pharmaceutical industries. Similarly, starch and starch syrups are traditional commodities in the food industry. Starch is also employed, after modification (oxidation or heating with a catalyst), as a surface sizing agent and a strength additive of chemical pulps (containing mainly cellulose) in the paper industry. In addition to chemical pulps, smaller amounts of so-called “dissolving pulps” are produced; the goal in their cooking and bleaching processes is to maximize the cellulose content of the product. These pulps are suitable for the production of a variety of cellulose derivatives and, for example, viscose fibers. In addition to cellulose and starch, there are other important polysaccharides (such as hemicelluloses, pectins, and “biogums”), which will become more important as renewable resources for new processes. In general, carbohydrates have a wide spectrum of applications, and several of their products already replace a part of similar chemicals manufactured in the petrochemical industry. On the other hand, carbohydrates form a unique polyfunctional group of materials that can be converted with synthetic chemistry techniques to respond to the growing needs of the future commercial life and chemical industry. It should be emphasized that cellulose is the most common organic polymer on the planet; thus, its structural part, glucose, is the most common organic molecule in nature.
Carbohydrate chemistry is today an established branch of organic chemistry. The main reason for this special position is the hydrophilic nature of the carbohydrates, which causes them to be generally insoluble in organic solvents so that their handling often requires “uncommon conditions”. In addition, sugars in particular are often seen as compounds that decompose easily and have a strong tendency to exist as mixed syrups that are difficult to investigate. The separation, purification, and structural study of unknown natural polysaccharides require special procedures that do not necessarily interest chemists outside this discipline. Another basic reason for the unique position of carbohydrate chemistry is the multiplicity of reactions that take place when starting from given initial compounds in the form of varying ring structures and the enhanced need for stereochemical evaluation in examining such reactions. Another special feature of carbohydrate chemistry is also the “unique system” of naming carbohydrates (about 3% of all organic compounds) that deviates in several aspects from the one used in other fields of organic chemistry. Clearly, our accumulated knowledge of carbohydrate chemistry is of fundamental importance, not only in the fields of organic and technical chemistry, but also for the understanding of biochemical processes and related areas, such as pharmacology.
![]()
2. Historical Background of Carbohydrate Utilization and Chemistry
2.1.The Era Before the 1800s
The instinctive or conscious use of carbohydrates must have begun in the early days of this planet’s civilization. Foods containing carbohydrates were processed in many ways quite early; for example, in ancient Babylon and Egypt, local people made beer and wine by fermenting grain starch or grape sugar (glucose). Clay tablets found in Sumer (now Southern Iraq) dated about 4000 B.C. contain mentions of a cereal-based fermented drink named “sikaru”. Moreover, besides starch, the use of sucrose and cellulose (see papermaking) for many purposes was known in early Far Eastern and Near Eastern cultures, from where this knowledge gradually spread to Europe. Developing uses for these “key material groups” until the 1800s will be discussed below; during the 1800s, carbohydrate chemistry gradually became a distinct branch of the emerging chemical knowledge.
Sucrose. The isolation of common sugar (sucrose or formerly known as saccharose) from the juice of sugarcane became an important milestone in the history of carbohydrates. The sugarcane plant probably originated from Northeastern India or the South Pacific; there is evidence from these areas (New Guinea ca. 10,000 B.C. and India ca. 6000 B.C.) of simple methods of making raw sugar. While it is still possible to see some wild sugarcane growing in India, the cultivation of this plant spread along with sugar use from India to China in 1800–1700 B.C. In the early days, sugar was separated for use as a yellowish syrup concentrate, which crystallized, on standing, into a brown mass of impure sucrose. In Sanskrit, the old cultural language in India, the word for sugar initially meant “brown sand”, which well describes the appearance of crushed raw sugar. On the other hand, the word “saccharose” is derived from the Sanskrit word “sarkhara”, which means “sweet”, from which follows the Latin word “saccharum”. Numerous Chinese texts from the 4th century B.C. describe in detail the process of concentrating the juice of sugarcane.
Arab traders brought the art of sugarcane cultivation to Mediterranean countries in ca. 1800 B.C. and developed methods for refining sugar mainly into products used to make early types of candy. Such candy was produced in large quantities, especially in Egypt in ca. 1000 B.C., where it was also utilized medicinally. The high nutritional value of sucrose was gradually recognized in many developing cultures. Pure, crystalline sucrose was finally described in India ca. 300 A.D., and its use extended along the caravan routes over North Africa into Spain. Importation of sucrose into Europe as an expensive sweetener increased during the 1300s and 1400s; many raw sugar refineries had already been established by the end of the 1400s. Since sugarcane could only be grown in tropical and subtropical climates, a search for alternate sugar-producing plants in temperate climates began in Europe. This led to selective breeding of the sugar beet in the late 1700s. Thereafter, the production of beet sugar as a substitute for cane sugar increased rapidly, especially while the Continental System (i.e., the British Navy blockade of Europe) established during the Napoleonic wars (1805–14) was in force. This meant the creation in Europe of a new and important branch of industry to utilize sources of carbohydrates.
Cellulose and paper. Many materials containing cellulose have long fulfilled human needs for dresses and housing. A widespread use of cellulose fiber as the main component in writing materials (such materials are collectively called “paper”) ...