
Furfural
An Entry Point of Lignocellulose in Biorefineries to Produce Renewable Chemicals, Polymers, and Biofuels
- 384 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Furfural
An Entry Point of Lignocellulose in Biorefineries to Produce Renewable Chemicals, Polymers, and Biofuels
About This Book
-->
There is a wide consensus that furfural, a renewable commodity currently obtained from lignocellulosic agro-residues with a production volume of around 300 kTon per year, is a key feedstock for leveraging lignocellulosic residues in future biorefineries. Several chemicals are already being manufactured from furfural due to its advantageous production cost. Furthermore, a vast number of others are also technically viable, to produce from oil.
This book compiles the vast existing information into relevant stages of transformations of furfural as renewable chemicals, biofuels and bioresins focusing on the relevant chemical and engineering aspects of processes to obtain them, including reactors and catalysis. It offers essential information for improving the economic and environmental viability of current commercial applications and upcoming future applications.
It should be of particular interests to graduate and advanced undergraduate students, as well as, engineers and academic researchers alike who are working in the field.
--> Contents:
- Preface
- About the Authors
- Chemistry of Furfural and Furanic Derivatives (Jesús Hidalgo-Carrillo, Alberto Marinas and Francisco J Urbano)
- Past, Current Situation and Future Technologies of Furfural Production (David Martín Alonso and Gianluca Marcotullio)
- Renewable Chemicals, Biofuels and Resins from Furfural:
- Furfuryl Alcohol and Derivatives (Pedro Maireles-Torres and Pedro L Arias)
- Tetrahydrofurfuryl Alcohol and Derivatives (Pedro Maireles-Torres and Pedro L Arias)
- Catalytic Transformations of Furfural and Its Derived Compounds into Pentanediols (Sibao Liu, Masazumi Tamura, Yoshinao Nakagawa and Keiichi Tomishige)
- 2-Methyl Furan and Derived Biofuels (Manuel López Granados, Inaki Gandarias, Iker Obregón and Pedro L Arias)
- 2-Methyl Tetrahydrofuran (MTHF) and Its Use as Biofuel (Iker Obregón, Inaki Gandarias and Pedro L Arias)
- Cyclopentanone and Its Derived Biofuel (Manuel López Granados)
- Levulinic Acid and γ-Valerolactone (Rafael Mariscal and David Martín Alonso)
- Amination of Furfural (Pedro Maireles-Torres and Pedro L Arias)
- On the Oxidation of Furfural to Furoic Acid (Michela Signoretto and Federica Menegazzo)
- Furan, Tetrahydrofuran and Other Furan-Derived Chemicals (Francisco Vila, Manuel Ojeda and Manuel López Granados)
- Catalytic Oxidation of Furfural to C 4 Diacids-Anhydrides and Furanones (Manuel López Granados)
- Biofuels and Chemicals from Furfural Condensation Reactions (Irantzu Sádaba and Manuel López Granados)
- Fuel Additives by Furfural Acetalisation with Glycerol (Manuel López Granados)
- Furanic Resins and Polymers (Thomas J Schwartz and Sikander H Hakim)
- Future Prospects and Main Challenges (Manuel López Granados and David Martín Alonso)
- Introduction to Chemical Reactors (José Miguel Campos-Martín)
- Introduction to Electrochemistry (María Retuerto and Sergio Rojas)
-->
--> Readership: Undergraduate and graduate students and academic and industrial researchers interested in the chemical transformations of furfural, biorefinery, biomass, biofuels, renewable chemicals. -->
Furfural;Biorefinery;Biomass;Biofuels;Renewable Chemicals0 Key Features:
- The first chapter is devoted to revising the organic chemistry and reactivity of furanic compounds
- Introduction to chemical reactors and electrochemistry as concepts are included as annexes, to help readers understand the subjects, as they are both frequently citied throughtout the book
Frequently asked questions
Information
Universidad de Córdoba, Campus de Rabanales,
Edificio Marie Curie, E-14071 Córdoba, España
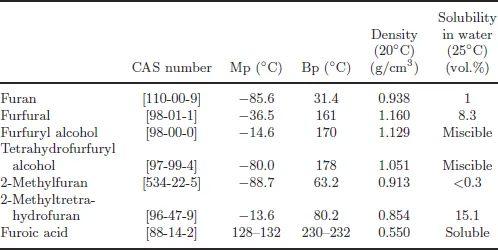
Derivative | Process reaction | Utilization |
Furfural | Xylosans dehydration | Natural precursor to a range of furan-based chemicals and solvents |
Furan | Furfural catalytic decarbonylation | Production of tetrahydrofuran and acetylfuran |
Furfuryl alcohol | Furfural catalytic hydrogenation | Production of resins and tetrahydrofurfuryl alcohol; intermediate in fragrances production, lysine and vitamin C |
Tetrahydrofurfuryl alcohol | Furfural catalytic hydrogenation | Solvent |
2-Methylfuran | Furfural and 5-methylfurfural decarbonylation | Solvent and monomer |
Furoic acid | Furfural oxidation | Synthesis of pharmaceuticals and perfumes |
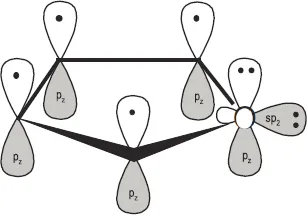
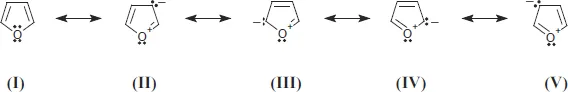
Table of contents
- Cover Page
- Title
- Copyright
- Preface
- About the Authors
- Contents
- Chapter 1 Chemistry of Furfural and Furanic Derivatives
- Chapter 2 Past, Current Situation and Future Technologies of Furfural Production
- Chapter 3 Renewable Chemicals, Biofuels and Resins from Furfural
- Chapter 3.1 Furfuryl Alcohol and Derivatives
- Chapter 3.2 Tetrahydrofurfuryl Alcohol and Derivatives
- Chapter 3.3 Catalytic Transformations of Furfural and its Derived Compounds into Pentanediols
- Chapter 3.4 2-Methyl Furan and Derived Biofuels
- Chapter 3.5 2-Methyl Tetrahydrofuran (MTHF) and its Use as Biofuel
- Chapter 3.6 Cyclopentanone and its Derived Biofuels
- Chapter 3.7 Levulinic Acid and γ-Valerolactone
- Chapter 3.8 Amination of Furfural
- Chapter 3.9 On the Oxidation of Furfural to Furoic Acid
- Chapter 3.10 Furan, Tetrahydrofuran and Other Furan-derived Chemicals
- Chapter 3.11 Catalytic Oxidation of Furfural to C4 Diacids-anhydrides and Furanones
- Chapter 3.12 Biofuels and Chemicals from Furfural Condensation Reactions
- Chapter 3.13 Fuel Additives by Furfural Acetalization with Glycerol
- Chapter 3.14 Furanic Resins and Polymers
- Chapter 4 Future Prospects and Main Challenges
- Annex 1 Introduction to Chemical Reactors
- Annex 2 Introduction to Electrochemistry
- Index