
Fundamentals of Electrothermal Atomic Absorption Spectrometry
A Look Inside the Fundamental Processes in ETAAS
- 416 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Fundamentals of Electrothermal Atomic Absorption Spectrometry
A Look Inside the Fundamental Processes in ETAAS
About This Book
This book provides the readers with the full basic knowledge necessary to understand, evaluate and develop critically any ETAAS analysis. The book covers comprehensively all aspects of the theoretical principles, routine and unusual instrumentation, overlapping possibilities with other techniques and different analytical characteristics of ETAAS at an averaged intermediate/high level. This is a good topic for a text book owing to the wide analytical possibilities of ETAAS in academic and industry laboratories. The book is written by a qualified expert with 30 years' experience working on different aspects of ETAAS.
The work guides the readers through an in-depth descriptive appraisal of the chemical and physical processes occurring in an ET atomiser. The work compares favourably with other books already published on this subject as this work shows an overview with some different perspectives, focusing mainly on the processes taking place during an ETAAS analysis. An ordered, rigorous and deep description is found in every chapter. The book would be adequate for undergraduate and graduate students in any course of analytical chemistry, researchers in analytical atomic spectrometry and analysts who routinely use ETAAS. Amateurs and specialists in this field will find a good support in the book.
Contents:
- Fundamental Spectroscopic and Analytical Processes in Atomic Absorption Spectrometry
- Atomizers
- Sample Introduction
- Radiation Sources, Spectral Dispersion, Isolation and Detection of Radiation
- Interferences: Types and Correction
- Chemical Modifiers
- Electrothermal Atomization Mechanisms: Theoretical and Practical Considerations
- Analytical Characteristics
Readership: Undergraduate and graduate students in any course of analytical chemistry, researchers in analytical atomic spectrometry, and analysts who routinely use ETAAS.
Key Features:
- The book differs from competing titles in the coverage and depth of the subject treated
- Topics related to the atomization mechanisms and interferences are treated in a deeply different perspective
- The author has published widely on relevant topics in ETAAS
Frequently asked questions
Information
Chapter 1
Fundamental Spectroscopic and Analytical Processes in Atomic Absorption Spectrometry
1.1.Introduction
1.2.Main Processes Occurring in an ET Atomizer: Atomization and Excitation
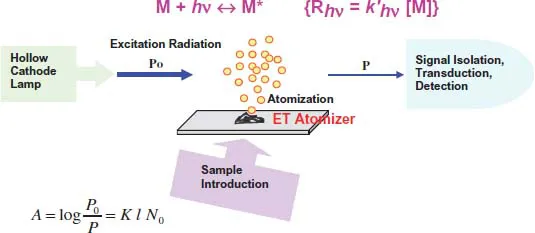
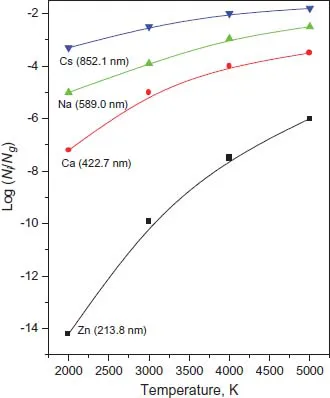
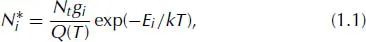
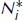
1.3.Spectroscopic Transitions
Table of contents
- Cover
- Halftitle
- Title
- Copyright
- Dedication
- Contents
- Preface
- Acknowledgements
- Chapter 1. Fundamental Spectroscopic and Analytical Processes in Atomic Absorption Spectrometry
- Chapter 2. Atomizers
- Chapter 3. Sample Introduction
- Chapter 4. Radiation Sources, Spectral Dispersion, Isolation and Detection of Radiation
- Chapter 5. Interferences: Types and Correction
- Chapter 6. Chemical Modifiers
- Chapter 7. Electrothermal Atomization Mechanisms: Theoretical and Practical Considerations
- Chapter 8. Analytical Characteristics
- Index