![]()
CHAPTER 1
Fantastic Metal Ions and How to Catch Them
Starting with a short survey of the metal ions, their size, charge and place in the periodic table, this chapter introduces ligands and the concept of their binding to metals and metal ions. Discover lobsters with teeth and why chelating ligands are so much more efficient at fishing out metals.
In the Periodic Table of Elements there are 92 naturally occurring building blocks of our world and most of them are metals. Some give us power, other give us wealth, and a fair number just keeps us and all other living beings on this planet alive and well. This book is about how to catch them, put them in the right places, make them do good things and sometimes to remove them from places where they might do harm.
1.1 The Tarnished Platinum Cyclist
Before starting in earnest we need to sort out some basic stuff, and we do this together with a rather questionable character, a young American who in July 1996, with the breath-taking speed of a racer cyclist, was on his way to Aix-les-Bains in south-east France. For the last few years, he had been pedalling himself into the world cycling elite, and now he could perhaps make his breakthrough in this, possibly the world's toughest athletic competition, the Tour de France. However, he did not make it to Aix, and a few months later it did not look like he would ever make it anywhere else on two wheels either. Lance Armstrong received frightening information from his doctors: he had testicular cancer and it had already spread to his lungs, abdomen and brain. He was given a less than 50% chance of survival.
Followers of Le Tour and, indeed, sports in general, all know how it ended. Armstrong recovered and went on to win a record-breaking seven consecutive Tour de France competitions from 1999–2005. Only to be disgraced and have them taken away years later when the long controversy of his alleged use of banned performance enhancing compounds finally reached the end of the road.
Why are we telling you this you wonder? Because what saved his life from the cancer was not only his tough physique and possibly some luck, but a very simple compound that is an easy first example of the type of chemistry that we will discuss in this book, the block-buster drug cisplatin.1 (To be clear, cisplatin is not a performance enhancing drug.)
Discovered by serendipity and then developed by much hard work by Barnett Rosenberg, Loretta Vancamp and co-workers at Michigan State University some 30 years earlier, it has a fascinating history of its own. Right now, however, we are concerned only with its simple components, two molecules of ammonia, two chloride ions and one platinum atom in the form of the ion with charge plus two, and how they stick together to form this amazing drug molecule, formally named cis-diamminodichloroplatinum(ii).
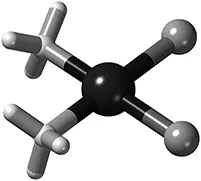
That is a bit of a mouthful, but start by noting that ammonia has the formula NH3 and is the same molecule that we find in some cleaning agents, that the chloride ion has charge minus one and is identical to the chloride ion in table salt, NaCl, and that platinum atoms are also found in catalytic converters for cars, but this one has two electrons removed to give the 2+ ion. Figure 1.1 represents how we like to picture the cisplatin molecule.
Figure 1.1The cisplatin anti-cancer drug has improved testicular cancer survival so much that the diagnosis is no longer a death sentence. It consists of two ammonia molecules (NH3 on the left side), a platinum 2+ ion in the middle (big and dark grey) and two chloride ions (Cl−) to the right. This picture tries to emphasise the 3D-form of the molecule (note how the nitrogen and chloride atoms sit in the corners of a square) while still showing the bonds as spokes. We call this form “square planar” when we discuss how the ligands bind to platinum.
Now, in your mind remove the bonds between Pt (platinum) and N (nitrogen), and between Pt and Cl (chlorine), and you will find five detached units, one metal ion and four other units that we call ligands† because they can bind to a metal ion. We will talk a lot about these ligands, so take note of this term.
But do not fear, we will not pepper the text with unnecessary jargon. We do need to name things to be able to talk about them though, imagine the sport pages without naming the individual footballers? Full of “this fellow”, “that fellow” and “the other fellow”. That would really be confusing! But this is a trap popular science occasionally falls into, sometimes to the extent of making the text unintelligible to layperson and specialist alike.
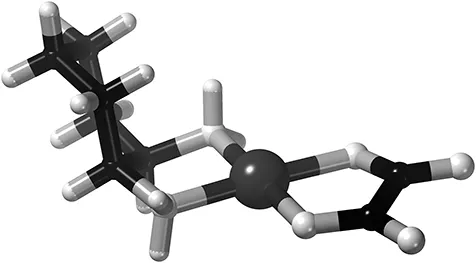
Back to cisplatin, which unfortunately will not cure all cancers, and sometimes a cancer may also develop resistance‡ against this drug. Then the doctor might suggest an alternative platinum-based drug, for example oxaliplatin (trade name elotaxin) which you can see in Figure 1.2.
Figure 1.2The anti-cancer drug oxaliplatin is used when cisplatin does not “bite”. The large ligand with many carbons (black) and hydrogens (white) to the left is a man-made molecule, whereas the ligand to the right with the oxygens (white) is the oxalate ion, as found in many fruits and vegetables, notably in rhubarb.
If you now remove the bonds between the metal and the ligands you will see that we are now left with two ligands instead of four, and one metal ion. This is because each ligand now binds to platinum 2+ with two atoms each, and Pt2+ still prefers having four bonds arranged in a flat “square planar” geometry.
Of course, there is a name for this as well, but we are afraid you will not find the following very logical. We call a ligand that binds to a metal with more than one atom a chelating ligand, and this comes from the Greek for claw, and what the coiners of this term thought about was the claws of a lobster as the ligand clearly pinches the metal and holds it fast. Fair enough, you might think, but then we call a ligand that can make a chelate a multi-dentate ligand, thus a ligand with many teeth, we count one for each atom that binds to the metal. And now it gets weird, as it is not clear if a lobster even has teeth, and if it does, they are certainly not sitting in the claws but seem somewhat surprisingly to be found in the stomach.
But here we are, and a claw with many teeth, incongruous as it may sound, is good if you want to hold on to a metal ion or catch it before it does harm or disappears before you can do something useful with it.
1.2 Chelation Catches the Criminal
One standard use of this chelate effect, the way in which a multi-dentate ligand is much better at binding and “catching” a metal ion than two similarly looking but mono-dentate (and thus non-chelating) ligands, is in scene-of-crime forensic analysis. Lance Armstrong may have acted immorally, at least in the eyes of the sporting world, but he didn't wave guns around, and there were no bullets to look for.
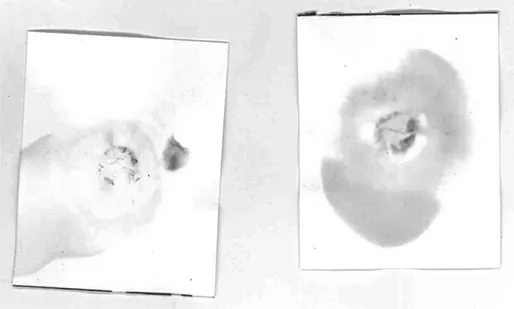
However, at a crime scene where shooting is suspected, this is exactly what you are searching for, and if you find a bullet hole, you will also stand a good chance of finding the actual bullet that can then subsequently be tied to a certain weapon. In some cases, it is not obvious which holes are bullet holes and which are not. Or worse, in some cases, it might be hard to know which are new bullet holes and which are old. That is why a small attaché case with a bullet-hole-test-kit is always carried to the scene of crime. The ligands in the single-use vials will both catch the lead or copper ions present in the bullet traces left in the hole and give a distinct colouring that can be detected on the spot (see Figures 1.3 and 1.4).
Figure 1.3Scene of crime bullet hole detection. The paper has been dosed with a detection liquid and pressed against the suspected bullet hole. A rose colour indicates the presence of lead and black-green indicates that copper has left a trace on impact. In black-and-white we can only see the circular traces. Photo credit: Lars Öhrström, holding the gun and detecting the bullets: Inspector Marie Brigantini, Swedish Police West.
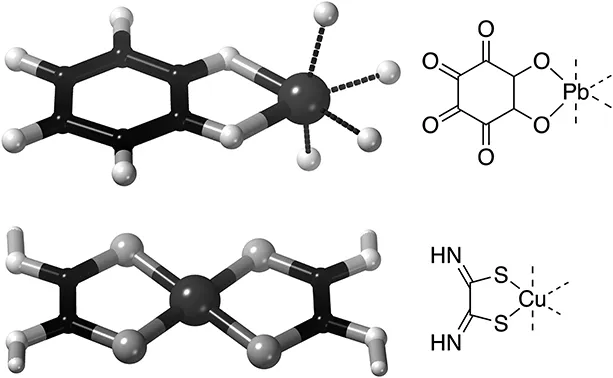
Figure 1.4Bullet hole detection: The bi-dentate ligands form chelates with the metal ions Pb2+ (left, only one ligand C6 O62− shown, the remaining bonds are represented by dotted lines) or Cu2+(centre, both dithiooxamide ligands Cu2+ are shown, this complex is charged 2+) giving coloured compounds that can be detected on the spot by the naked eye, allowing the forensic examiner to go on to find the bullet. Right: The corresponding so-called Lewis-structures. These will occasionally be displayed for the benefit of those needing some more detailed information.
Having said that, nothing is ever as simple as implied in the high-heeled-CSI shows on the telly. Ambiguous results, false positives and failure for the reagents to be specific enough, or to react with a low enough concentration of the suspected criminal residue, are some problems facing both the investigating officers and the chemist developing the methods.
1.3 What About the Metals?
So, multi-dentate ligands can catch metal ions, but there are so many different metals, are there no differences between them? Of course there are. Copper for example, likes sulfur (S) a lot, and it is not a coincidence that the Cu2+ ion is detected with a sulfur containing ligand in the bullet-hole-test. Calcium ions (Ca2+) on the other hand, do not have much affinity, as we say, for ligands with sulfur, as their bonding atoms prefer oxygen, which is their natural surroundings in bones, teeth and marble for example. The metal ions also come in different sizes, the Li+ ion, very important in the treatment of some forms of bipolar disorder, is very small, 73 picometres, whereas the Pb2+ ion, a rather nasty fellow, has almost twice this radius, 143 picometres, that is 0.000000000143 millimetres.
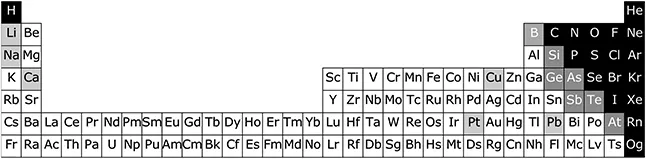
It will not be necessary to memorise the Periodic Table to follow the remainder of the book and for now, a quick look at Figure 1.5 will suffice.
Figure 1.5The Periodic Table of Elements.§ On the black background are the non-metallic elements, the metals are on the white background, and on the grey background with white text are the elements that sometimes behave as metals and sometimes not. The six metals we have already encountered, lithium (Li), sodium (Na), calcium (Ca), copper (Cu), platinum (Pt) and lead (Pb) have black symbols on a grey background.
There are so many metals, but still fewer than footballers in the Premier League. We will be mostly concerned with some of the “stars” such as gold (Au), but also lesser known metals, such as gadolinium (Gd) might find their way into the stories. We do not want to be biased though, every naturally occurring element is important in some way. As a student once said “all elements are created equal and should be treated with respect” (this particular chemistry class had been preceded by a diversity workshop).
One thing to take home from Figure 1.5 however, is that elements appearing in the same column tend to have similar properties, that is kind of why we call it the periodic table. Therefore, l...