
- 648 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Organic Catalysis for Polymerisation
About this book
In recent years polymerisation using organocatalysts has become an appealing alternative to more traditional metal-based catalysts. Conferring numerous advantages including low cost and ease of use, as well as the ability to precisely control the synthesis of advanced polymer structures, organocatalysts are increasingly used in polymer synthesis. Organic Catalysis for Polymerisation provides a holistic overview of the field, covering all process in the polymer synthesis pathway that are catalysed by organic catalysts. Sub-divided into two key sections for ease of use, the first focuses on recent developments in catalysis and the applications of catalysts to the full range of polymerisations that they have been utilised in; the second concerning monomers, arranges the field by monomer type and polymerisation mechanism. The book will therefore, provide a complimentary view of the field, providing both an overview of state-of-the-art catalyst development and also the best methodologies available to create specific polymer types. Edited by leading figures in the field and featuring contributions from researchers across the globe, this title will serve as an excellent reference for postgraduate students and researchers in both academia and industry interested in polymer chemistry, organic chemistry, catalysis and materials science.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Email: [email protected]
1.1 Introduction
1.2 Definition of ZROP
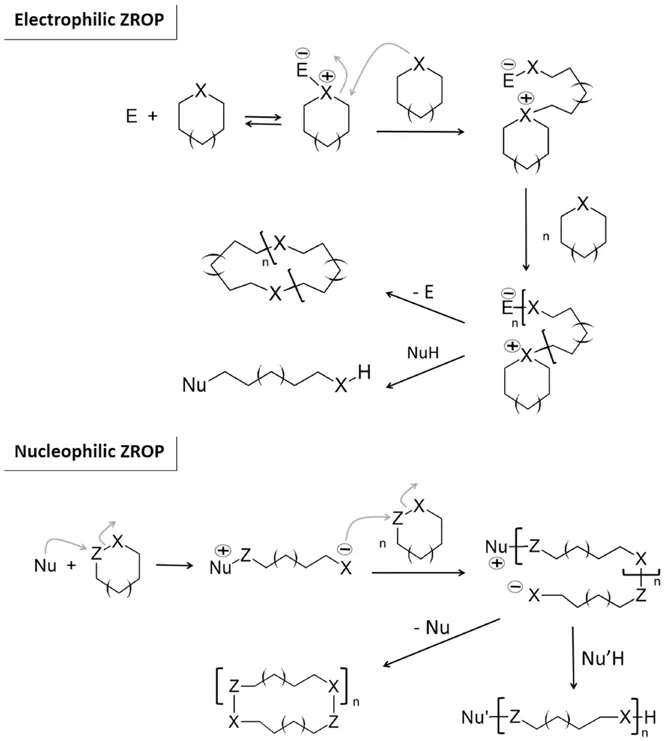
1.2.1 Pyridine-based Initiation
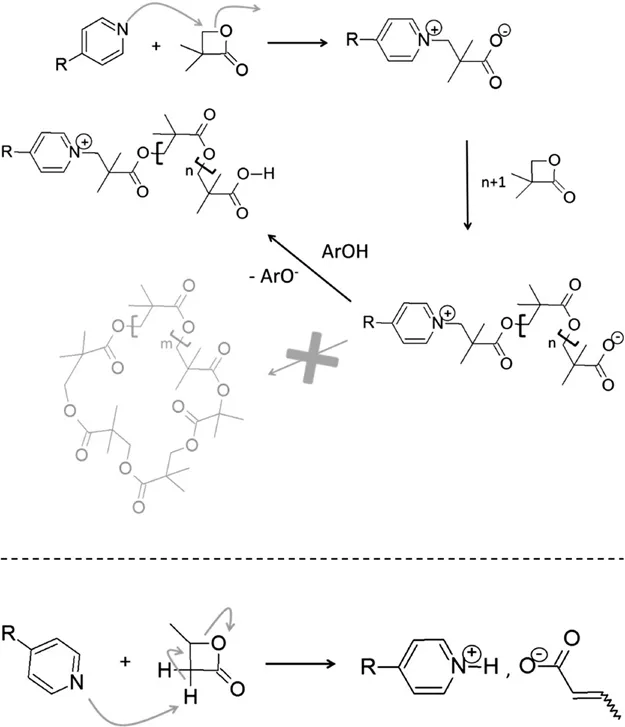
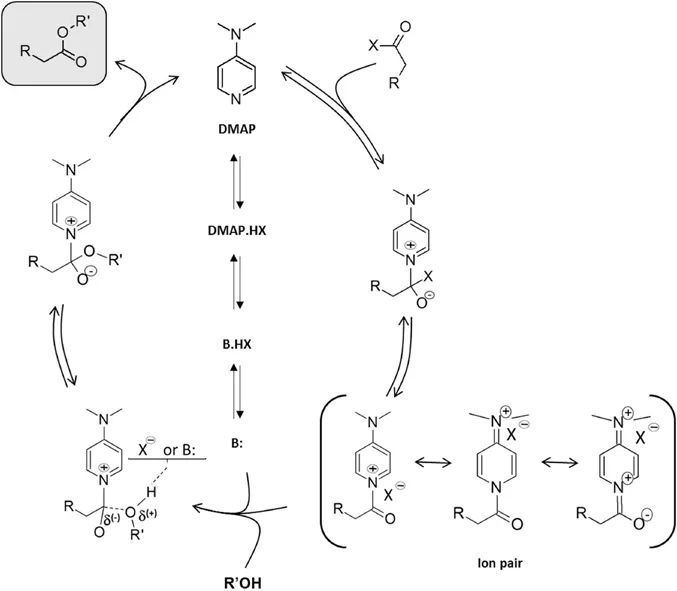
Table of contents
- Cover
- Title Page
- Copyright Page
- Preface
- Contents
- Chapter 1 Nucleophilic Catalysts and Organocatalyzed Zwitterionic Ring-opening Polymerization of Heterocyclic Monomers
- Chapter 2 Ring-opening Polymerization Promoted by Brønsted Acid Catalysts
- Chapter 3 Bifunctional and Supramolecular Organocatalysts for Polymerization
- Chapter 4 Base Catalysts for Organopolymerization
- Chapter 5 Ring-opening Polymerization of Lactones
- Chapter 6 Organic Catalysis for the Polymerization of Lactide and Related Cyclic Diesters
- Chapter 7 ROP of Cyclic Carbonates
- Chapter 8 Metal-free Polyether Synthesis by Organocatalyzed Ring-opening Polymerization
- Chapter 9 Ring-opening Polymerization of N-carboxyanhydrides Using Organic Initiators or Catalysts
- Chapter 10 Organocatalytic Ring-opening Polymerization Towards Poly(cyclopropane)s, Poly(lactame)s, Poly(aziridine)s, Poly(siloxane)s, Poly(carbosiloxane)s, Poly(phosphate)s, Poly(phosphonate)s, Poly(thiolactone)s, Poly(thionolactone)s and Poly(thiirane)s
- Chapter 11 Organopolymerization of Acrylic Monomers
- Chapter 12 Organocatalyzed Step-growth Polymerization
- Chapter 13 Organocatalyzed Controlled Radical Polymerizations
- Chapter 14 Organocatalysis for Depolymerisation
- Chapter 15 Organic Catalysis Outlook: Roadmap for the Future
- Subject Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app