![]()
CHAPTER 1
Vitamin E: Structure, Properties and Functions
Etsuo Niki*a and Kouichi Abeb
a University of Tokyo, Research Center for Advanced Science and Technology (RCAST), Komaba, Tokyo 153-8904, Japan
b SSCI Laboratories, Faculty of Pharmacology, Musashino University, Nishi-Tokyo-Shi, Tokyo 202-0023, Japan
*E-mail:
[email protected] Vitamin E is the collective name for lipophilic, naturally occurring compounds whose molecular structure is comprised of a chromanol ring with a side chain located at the C2 position and includes four tocopherols and four tocotrienols. Vitamin E, discovered as a dietary factor essential for normal reproduction, is now accepted as a major free radical scavenging antioxidant in humans and protects biological molecules from detrimental oxidative modifications. The structures and properties of vitamin E homologues and their sources, functions, and applications are summarized.
1.1 Introduction
In 1922, Evans and Bishop demonstrated the existence of a hitherto unrecognized dietary factor essential for normal reproduction in the rat.1 It was accepted at that time that the most striking function of vitamin E was to provide a normal gestation in a pregnant rat to prevent the resorption of the embryos which invariably occurred in its absence.2 This unknown dietary factor X was found to be present in green lettuce, dried alfalfa leaves, wheat, and oats. Evans isolated the factor X from wheat germ oil, provided the chemical formula C29H50O2 and proposed the name α-tocopherol in 1936.3 The structural formula for α-tocopherol was provided by Fernholz in 1938.4 Tocotrienols were discovered much later than tocopherol and named in the early 1960s.5,6
Olcott found that the lipid fractions of vegetable oils contained antioxidants against the oxidative deterioration of lard.7 Since then, it has been unequivocally demonstrated that vitamin E acts as an essential antioxidant in vivo as well as in vitro and plays an important role in the prevention of detrimental oxidative damage of biological molecules.8,11 More recently, the non-antioxidant functions of vitamin E, including cellular signaling, gene regulation, membrane processes, and nerve functions, have also received much attention.12,14 However, many issues are still controversial and remain to be elucidated. Sound information based on solid chemical evidence is essential for understanding the role of vitamin E in vivo as well as in vitro.
1.2 Homologues: Nomenclature and Structure
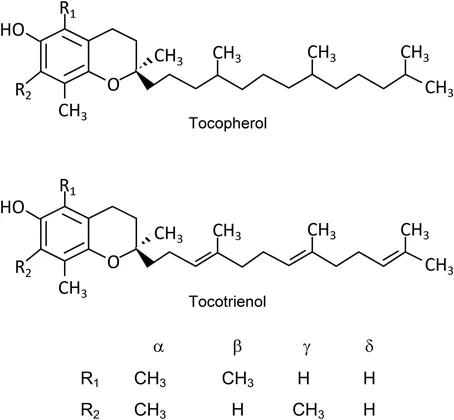
Vitamin E is a plant-derived, lipid-soluble substance whose molecular structure is comprised of a chromanol ring with a side chain located at the C2 position. Vitamin E refers to a group of eight different compounds: α-, β-, γ-, and δ-tocopherols and the corresponding four tocotrienols. The four tocopherols have a saturated phytyl side chain, while tocotrienols have an unsaturated isoprenyl side chain containing three double bonds at C3′, C7′, and C11′. The double bonds of tocotrienols' side chains at C3′ and C7′ have a trans-configuration. The α-, β-, γ-, and δ-forms differ with respect to the number and position of methyl groups on the chromanol ring. The α-forms of tocopherol and tocotrienol have three methyl groups at the C5, C7, and C8-positions of the chromanol ring, while the β- and γ-forms have two and the δ-forms have one methyl group as illustrated in Figure 1.1.
Figure 1.1Chemical structure of vitamin E homologues.
In addition to tocopherols and tocotrienols, tocomonoenols and tocodienols containing a single and two double bond unsaturation, respectively, have also been found in nature. For example, a tocomonoenol with a single double bond at carbon 11′, 2,5,7,8-tetramethy1-2-(4′,8′,12′-trimethyltrideca-11′-enyl)-6-chromanol, was isolated from palm and rice bran oils.15 Since then, several groups have detected tocomonoenols in plants and plant foods, such as α-tocomonoenol in palm oil,16,22 pumpkin seed oil (Cucurbita pepo L.),23 and sunflower oil (Helianthus annuus),24 γ-tocomonoenol in pumpkin seed oil,22,23 δ-tocomonoenol in kiwi (Actinidia chinensis),25 and β-, γ-, and δ-tocomonoenol in the leaves of Kalanchoe daigremontiana and Phaseolus coccineus.26 A tocomonoenol with an unsaturation at the isoprenoid-chain terminus was also found in the tissues of salmon.27 Furthermore, tocodienols with two double bonds at carbon 7′ and 11′ were identified in palm oil.16,21
Tocopherols contain three chiral carbons, one at C2 in the chromanol ring and two in the side chain at C4′ and C8′. Naturally occurring α-tocopherol contains chiral carbons in the R-conformation, 2R, 4′R, and 8′R-α-tocopherol. α-Tocotrienol has one chiral center at C2 in the chromanol ring and natural tocotrienols occur as the R-isoform. On the other hand, the chemical synthesis of α-tocopherol produces an equimolar mixture of eight different stereoisomers: RRR, SRR, RSR, RRS, RSS, SSR, SRS, and SSS. The synthetic α-tocopherol is called all-rac-α-tocopherol. An equimolar mixture of RRR-α-tocopherol and SRR-α-tocopherol is called 2-ambo-α-tocopherol. The IUPAC names of RRR-α-tocopherol and RRR-α-tocotrienol are (2R)-2,5,7,8-tetramethyl-2-[(4R,8R)-(4,8,12-trimethyltridecyl)]chroman-6-ol and (2R)-2,5,7,8-tetramethyl-2-[(3E,7E)-4,8,12-trimethyltrideca-3,7,11-trienyl]-3,4-dihydrochromen-6-ol, respectively.
Ester forms of tocopherol and tocotrienols, including acetate, nicotinate, succinate, and phosphate, have been prepared and their action and potential applications have been studied. Vitamin E is easily oxidized when subjected to heat, light, and alkaline conditions, but esters are less susceptible to oxidation and therefore more appropriate for food, cosmetic, and pharmaceutical applications compared to the free form. Polyethylene glycol conjugates of tocopherols and tocotrienols have the ability to form miscible micelles in water due to amphiphilic properties and enhance bioavailability in animals and humans via improving their water solubility and absorption.28 It was reported that RRR-α-tocopheryl polyethy...