![]()
CHAPTER 1
Introduction
1.1 Introduction
Each year, millions of people suffer from spinal cord injury and diseases such as myocardial infarction, diabetes, and leukemia. In the past, therapeutic approaches have been limited to the removal of injured parts by surgery and medical treatment.
Human pluripotent stem (hPS) cells have high differentiation ability relative to adult stem cells such as adipose-derived stem cells and bone marrow-derived stem cells. Several studies have demonstrated that hPS cells can be differentiated into specific cell lineages derived from three germ layers.1,2 Thus, hPS cells are a promising source for the replacement of damaged or lost cells in regenerative medicine. However, it is necessary to control the proliferation and differentiation of stem cells in xeno-free culture conditions for the clinical use of stem cells. In this case, cell culture biomaterials play an important part in the stem cell fate of proliferation as well as the stem cell fate of differentiation into specific lineages of cells that are going to be used for drug discovery and regenerative medicine.
1.2 Stem Cells
Stem cells are capable of self-renewal, proliferation, and differentiation to various cell lineages, making them advantageous for regenerative medicine applications. Importantly, self-renewal and cellular proliferation are not synonymous, because the former term encompasses both the differentiation and future mitotic potential of the daughter cells in addition to cell division.3–5
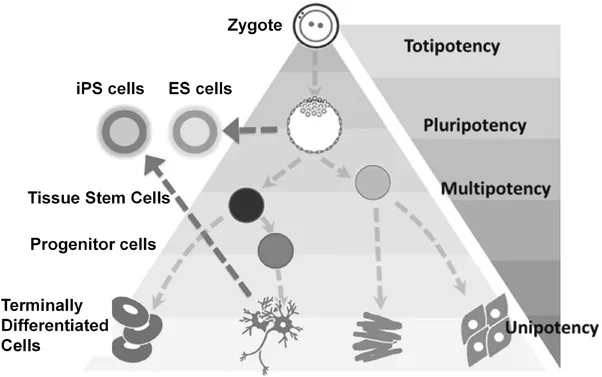
Depending on the type and maturity of stem cells in the tissue, stem cell potency and capacity for self-renewal can be varied.6 There are two main types of stem cells, embryonic and non-embryonic cells. Embryonic stem (ES) cells are pluripotent and ES cells can differentiate into the cells derived from all three germ layers (ectoderm, mesoderm, and endoderm). Non-ES cells are multipotent. Their potential to differentiate into different cell types seems to be more limited3 and more importantly, multipotent stem cells have an aging problem (limited proliferation) which ES cells do not have (infinite proliferation). The differentiation potential can be classified into four levels: totipotent, pluripotent, multipotent, and unipotent stem (progenitor) cells (Figure 1.1).7
Figure 1.1 Hierarchical potential of stem cell development.7 Adapted from ref. 7 with permission from Springer Nature.
Totipotent stem cells can differentiate into embryonic and extra-embryonic cell types, which can construct a complete and viable organism. Totipotent cells are produced from the fusion of an egg and a sperm cell. The fertilized egg and the cells produced by the first few divisions of the fertilized egg are totipotent. Totipotent stem cells give rise to somatic stem/progenitor cells and primitive germ-line stem cells.8
Pluripotent stem cells can differentiate into nearly all cell types of the adult organism, because they have the ability to differentiate into the cells derived from three germ layers: endoderm, mesoderm, and ectoderm.9,10
ES cells, which are pluripotent stem cells, are derived from totipotent cells of the early mammalian embryo and are capable of differentiating into cells representing the three embryonic germ layers, namely ectoderm, mesoderm, and endoderm or any of more than 100 cell types present in the adult body, and are characterized by self-renewal, immortality, and pluripotency. ES cells are unlimited and show undifferentiated proliferation in vitro.11–15
Multipotent stem cells can differentiate into a number of cells, but only those of a closely related family of the cells. These are stem cells but can only differentiate into a limited number of cell types. For example, the bone marrow contains multipotent stem cells that give rise to all the cells of the blood but not to other types of cells (hematopoietic stem cells), as well as bone marrow-derived stem cells, which are typical mesenchymal stem (MS) cells. Adipose tissue also contains a source of multipotent stem cells (adipose-derived stem cells).16
Unipotent stem (progenitor) cells denote a state lineage plasticity to differentiate into only a few cells.17 Unipotent stem cells are those such as lymphoid or myeloid stem cells. The corneal epithelium is a squamous epithelium18 that is constantly renewing and is regarded as an unipotent stem cell.19 Unipotent progenitor cells can produce only one cell type (their own), but have the property of self-renewal, which distinguishes them from non-stem cells. Most epithelial tissues self-renew throughout adult life due to the presence of unipotent progenitor cells.20
There are ethical difficulties regarding the use of human embryos, as well as the problem of tissue rejection following transplantation in patients. One way to circumvent these issues is the generation of pluripotent cells directly from the patient’s own cells.21 Somatic cells can be reprogrammed by transferring their nuclear contents into oocytes22 or by fusion with ES cells,23,24 indicating that unfertilized eggs and ES cells contain factors that can confer totipotency or pluripotency to somatic cells.
Through analyzing the gene expression profiles of ES cells, many highly expressed genes in ES cells have been identified. In 2006, Yamanaka and his colleague21 successfully introduced 24 transcription factors (pluripotent genes) that are highly expressed in ES cells into the fibroblast cells derived from fetal mice. Surprisingly, some ES-like colonies appeared in the culture dish within 2 weeks of retroviral infection. Moreover, these ES-like cells could be propagated in vitro and resemble ES cells morphologically after many cell passages.14 They found ultimately that four transcription factors including Oct4, Sox2, Klf4, and c-Myc25 (nowadays, Oct4, Sox2, and Klf4 or less transcription factor26) were essential for converting the fibroblast cells into induced pluripotent stem (iPS) cells by reducing the factors one by one in the process of retroviral infection. The iPS cells were created by inducing the specialized cells to express genes that were normally present in ES cells and that control cell functions.27 However, iPS cells could be successfully derived from the differentiated somatic cells simply based on the morphology changes and no genetic selection was needed, which indicated that human somatic cells without genetic modification could be reprogrammed successfully.28,29
Since their discovery in the mid-2000s, newer generations of iPS cell lines have been created through various non-integrating reprogramming strategies, such as approaches using mRNA,30 episomes,31,32 minicircles,33 piggyBac transposons,34 recombinant proteins35 or Sendai virus.36,37
In 2012, Shinya Yamanaka and Sir John Gurdon were awarded a Nobel Prize for their combined efforts in discovering that “mature cells can be reprogrammed to become pluripotent”. iPS cells were created by inducing the specialized cells to express pluripotent genes that were normally present in ES cells and that controlled cell functions. In addition, iPS cells have advantages over ES cells: iPS cells are capable of generating autologous and non-immunogenic patient-specific therapies and can more easily provide cell-based disease models from genetically predisposed patients. These newer generations of iPS cell lines avoid the tumorigenicity risks associated with the genomic integration of reprogramming factors and are a powerful way of creating patient- and disease-specific cell lines for rese...