eBook - ePub
The Lung
Developmental Morphogenesis, Mechanobiology, and Stem Cells
This is a test
- 204 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
The Lung
Developmental Morphogenesis, Mechanobiology, and Stem Cells
Book details
Book preview
Table of contents
Citations
About This Book
With detailed scientific background and up-to-date research, this book examines recent developmental and cell biology, mechanobiology and stem cell biology discoveries to help provide a better understanding of lung development, repair and regeneration.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access The Lung by Ahmed El-Hashash, Eiman Abdel Meguid in PDF and/or ePUB format, as well as other popular books in Medicine & Pulmonary & Thoracic Medicine. We have over one million books available in our catalogue for you to explore.
Information
Publisher
WSPCISBN
9789813277083
Topic
MedicineSubtopic
Pulmonary & Thoracic MedicineChapter 1
Lung Pattern Formation and Development
Abstract
The major function of the lung in different organisms is to perform an efficient exchange of gas with the atmosphere. The thickness of the surface of gas diffusion of the mature lung is 1 micron in humans. However, this produces a surface area of 70 square meters, which is equivalent in size to a modern tennis court or the wing surface of a small aircraft. The lung has a complex organization within the chest as a honeycomb-like structure that comprises a network of extensively branched ducts that function to conduct air to and from the alveolar gas exchange surface. This occurs in a configuration that remarkably increases the surface that facilitates gas exchange between blood and air, while enabling maximally efficient packing of this surface within the chest cavity. This complex structure of the lung is developed sequentially by early branching of the epithelial tube and later on by the process of the septation of terminal air sacs. In addition, the development of pulmonary vasculature that occurs in conjunction with epithelial branching morphogenesis acts to facilitate the transport of respiratory gases to and from the developing alveolar surface. In conjunction with these developmental processes, the development of airway smooth muscle (ASM) takes place during early lung morphogenesis, and its contraction may function to regulate the growth of the lung. Any perturbation of these tightly regulated developmental processes can lead to the formation of abnormal lung structure, gas exchange deficiency and/or respiratory failure. Such disruption of normal lung growth and development is clinically exemplified in many cases, such as premature human delivery, bronchopulmonary dysplasia, or congenital lung defects or disorders. This chapter will describe different phases of lung development, genetic control of the formation pattern of early lung anlagen, distal airway branching morphogenesis, and the alveolar septum formation and its regulatory factors and molecular mechanisms as well as the development of various lung-specific cell types.
Keywords: Lung; developmental phases; pseudoglandular; canalicular; saccular; alveolar; pattern formation; branching morphogenesis; lung analgen.
1.1Introduction
Throughout nature, branched networks are ubiquitous and particularly exist in various tissue types that require large surface area within a restricted volume. A wide range of tissues with a branched architecture, including the lung, kidney, vasculature, mammary gland and nervous system, function to exchange gases, fluids and information throughout the body of an organism. The generation of different branched tissue types requires a proper control of various biological processes, such as the specification, initiation and elongation of the branch site. Despite their complexity, the branching events in different organ types normally require the coordination of many cells to build a network of tissues for material exchange (Spurlin and Nelson, 2017).
The development of the respiratory system occurs through a series of steps that begin with the division of the common foregut tube into the respiratory endoderm anteriorly and the esophagus posteriorly. The respiratory tract undergoes excessive branching to form the proximal conducting airways, followed by distal septation that is able to generate the gas exchange units, called the alveoli. In addition, the formation of the lung is dependent on the development of the bronchial vascular system and the pulmonary vascular system that occurs simultaneously.
The steps in lung development are dependent upon inductive cues and crucial interactions between the pulmonary epithelium and the surrounding mesenchyme. The absence of these reciprocal interactions can lead to anatomical anomalies. The regulatory mechanisms and many aspects of the activity of lung mesenchymes are still not well known (Morrisey et al., 2013).
The human lungs arise from the laryngotracheal groove, which evaginates into the surrounding splanchnic mesenchyme. They originate from the anterior surface of the primitive foregut at 5 weeks of gestation in humans and continue to grow for several months after birth (recently reviewed by Warburton et al., 2010; Rankin and Zorn, 2014; Schittny, 2017; Chen and Zosky, 2017).
During the fourth week of embryogenesis, the larynx, trachea, bronchi and lungs begin to form from the respiratory bud, which starts to appear ventral to the caudal portion of the foregut. By the end of the fourth week, a pair of primary bronchial buds evaginate from the laryngotracheal groove (Figure 1). During the fifth week, the left bud gives two secondary bronchial buds while the right bud gives three secondary bronchial buds. These secondary bronchial buds give rise to the lung lobes. Over the following week, the secondary buds branch into tertiary buds, which number ten on each side. From the sixth week to the sixteenth week, the major elements forming the lungs appear, except the alveoli. From week 16 to week 26, the bronchi increase in size and lung tissue vascularises highly. Bronchioles and alveolar ducts also develop. Alveolarization begins at around 20 weeks in humans, and continues postnatally till 7 years of age (Figures 1 and 2; Warburton et al., 2010; Schittny, 2017).
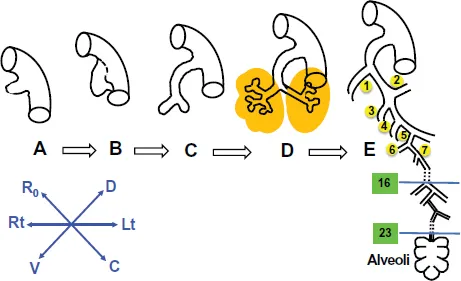
Figure 1.Lung pattern formation and development. (A) The primitive lung anlage emerges as the laryngotracheal groove from the ventral surface of the primitive foregut at 5 weeks’ gestation in the human. (B) The primitive trachea separates dorsoventrally from the primitive esophagus as the two primary bronchial branches arise from the lateral aspects of the laryngotracheal groove at 5 or 6 weeks’ gestation in the human. (C) The embryonic larynx and trachea with the two primary bronchial branches are separated dorsoventrally from the embryonic esophagus at 6 weeks in the human. (D) The primitive lobar bronchi branch from the primary bronchi at 7 weeks in the human. (E) A schematic rendering of the airway at term in the human. The stereotypical first 16 airway generations are complete by 16 weeks in humans; between 16 and 24 weeks, further branching is nonstereotyped. Alveolarization begins about 20 weeks in humans and is complete by 7 years of age at the earliest. (After West, Burri, Warburton, and others).
In the human lung, 23 generations of airway branching are found (Figure 1). The first sixteen generations branch stereotypically. This stage of branching is completed by 16 weeks, while the remaining seven generations are completed almost at 20–24 weeks. Differentiated type I alveolar epithelial cells, which are involved in the process of alveolar blood gas exchange, together with the type II alveolar epithelial cells that produce pulmonary surfactants are formed during lung development. The surfactant reduces the surface tension at the air–alveolar surface, and therefore helps the terminal saccules to expand (Warburton et al., 2010; Schittny, 2017).
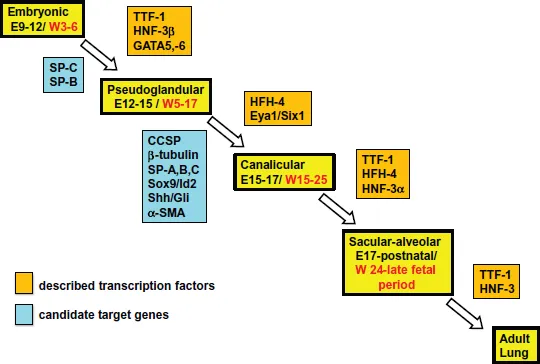
Figure 2.Timeline of the phases of lung development and some key transcription factors with their specific targets affecting each phase. Timeline for lung development in the mouse is shown in black text in days [E], while for human it is shown in red text in weeks [W]. Despite identical phases of lung development, the timing for each phase is markedly different between mice and human. Transcription factors are shown in an orange box, and their known targets, at the same phase of development, are shown in a blue box.
1.2Brief Overview of the Human Respiratory System
The respiratory system consists of two divisions: the upper respiratory tract, which consists of nose and pharynx, and the lower respiratory tract, which consists of larynx, trachea, right and left bronchi, and lungs.
The bronchial tree consists of different parts, such as the primary (main) bronchus, secondary (lobar) bronchus for each lobe, tertiary (segmental) bronchus for each segment, conducting (lobular) bronchiole (1 mm; no cartilage), terminal bronchiole, respiratory bronchiole and alveolar duct as well as the alveoli.
Air first enters our body through the nose or mouth, and then passes through the nasopharynx into the oral pharynx. Then, it passes through the glottis and the trachea into the right and left bronchi, which branch into bronchioles, each of which terminates in a cluster of alveoli. There are 300 million alveoli, which provide a surface area of 160 m2 for gas exchange to take place, in a pair of adult lungs.
The pharynx is divided into three parts: nasopharynx, oropharynx and laryngopharynx. The nasopharynx lies posterior to the nasal cavity above the soft palate. The pharyngeal tonsil is in the roof of the nasopharynx. On the lateral wall of the nasopharynx is the opening of the auditory tube. This part of pharynx has a respiratory function as it is a passage for air. The oropharynx is the oral part of the pharynx and lies posterior to oral cavity, extending from below soft palate to the level of vertebra C3. The floor of the oropharynx is formed by the posterior one-third of the tongue. On the lateral wall of the oropharynx are the palatine tonsils. The oropharynx is the part of the pharynx that is a common pathway for air and food. The laryngopharynx is the part of the pharynx that extends from the oropharynx above and continues as oesophagus. It lies behind the opening into the larynx and extends to the level of C6. This part of the pharynx is also a common pathway for air and food.
The human trachea is normally 13 cm long and 2.5 cm in diameter. It has a fibroelastic wall made of U-shaped bars of hyaline cartilage, which keep the lumen patent. The posterior free ends of the tracheal cartilages are connected by a smooth muscle, the trachealis muscle. The trachea commences in the neck below the cricoid cartilage (the 6th sixth cervical vertebra) and ends at the sternal angle by dividing into the right and left bronchi. The bifurcation is called the carina. During deep inspiration, the carina descends to the level of the sixth thoracic vertebra. An enlargement of the thyroid gland in the neck can cause gross displacement or compression of the trachea, while a dilatation of the aortic arch (aneurysm) can also compress the trachea.
Bronchial asthma is a common condition that is characterized by a clear narrowing of the airways. Asthma is caused by a remarkable smooth muscle cell contraction, and an edema of the mucosa as well as accumulated mucus in the lumen of both the bronchi and bronchioles. These pathological changes occur as the result of the rapid histamine release that markedly affects both the caliber and tone of blood vessels (Holgate et al., 2015). In addition, the difficulty of expiration in asthma is most likely because of the bronchioles, which are normally opened during inhalation of air and also remain open during exhalation of air to permit a rapid air outflow (Holgate et al., 2015).
Lungs have a half-cone shape, with a base, apex, two surfaces (costal and mediastinal) and three borders (anterior, inferior and posterior). The lung base sits on the diaphragm, while the lung apex projects above the first rib and into the root of the neck. The hilum is where structures enter and leave the lung. The heart and major vessels indent the mediastinal surface of the lung.
The lungs are surrounded by pleura. Parietal pleura covers the diaphragm, mediastinum and the thoracic wall, while visceral pleura covers the outer surfaces of the lungs and dips into its fissures. The pleural cavity localizes between these two layers and is filled with pleural fluid secreted by the pleural membranes. The pleural cavity functions to provide lubrication, thereby preventing friction during breathing. Parietal pleura is reflected from the mediastinum to the lung to form the visceral pleura. Such an arrangement creates recesses in the pleural cavity between the layers of the pleural reflections.
The parietal pleura consists of the cervical pleura, the costal pleura, the diaphragmatic pleura and the mediastinal pleura. The cervical pleura extends up into the neck, lining the undersurface of the supra-pleural membrane. It reaches a level 1–1.5 inch (2.5–4 cm) above the medial third of the clavicle. The costal pleura lines the inner surfaces of the ribs, the costal cartilages, the intercostal spaces, the sides of the vertebral bodies, and the back of the sternum. The diaphragmatic pleura covers the thoracic surface of the diaphragm. The mediastinal pleura forms the lateral boundary of middle mediastinum.
1.3Phases of Lung Development
Lung development has been divided into five phases, the embryonic, pseudoglandular, canalicular, terminal saccular and alveolar (Warburton et al., 2010; Schittny, 2017; Chen and Zosky, 2017, Figures 2 and 3 for some histological stages of murine lung development).
During early embryonic development, many signals from the developing mesenchymal tissues act to pattern the development of foregut endoderm in an anterior–posterior manner. This patterning occurs first into broad domains and then into more specific and restricted regions where the anlages of the prospective organ will bud into the neighboring mesenchymal tissue (Warburton et al., 2010). In addition, morphological movements of the developing embryo during embryogenesis can expose the growing endoderm to changing patterns of mesenchymal cell-specific gene expression. Consequently, specific regions of the developing foregut can experience gradients of different morphogens at specific times of foregut development. These characteristically well-organized signaling environments can function to tightly regulate the normal patterning of the developing foregut endoderm. The timing, strength and extent of exposure of these developmental signals can indeed have a significant impact on the differentiation and specification of organ-specific foregut endodermal progenitor cells (Warburton et al., 2010). Identification and characterization of signals and pathways that can promote lung endodermal development are, therefore, crucial to determine the generation of many specific types of epithelial cells from undifferentiated pluripotent stem cells, which can provide both basic scientific knowledge and tools for the treatments of a wide range of lung diseases.
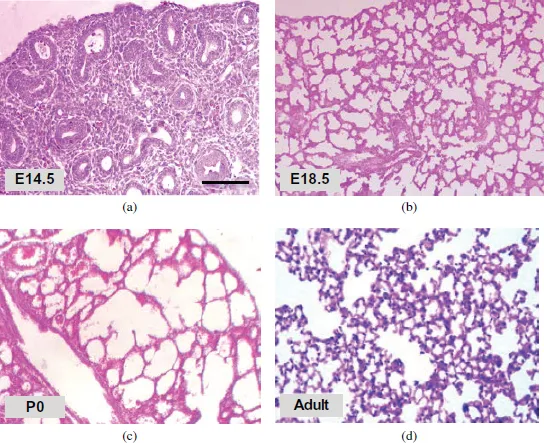
Figure 3.Hematoxylin and Eosin staining shows histology of murine lung at different developmental stages. (a–d) Embryonic mouse lung develops from pseudoglandular stage (a; E14.5) to canalicular stage, and further terminal sac stage (b–c; e18.5 and P0). Neonatal mouse lungs undergo alveolarization, resulting in the formation of many septa. A mature honeycomb-like structure with alveoli surrounding alveolar ducts conferring normal re...
Table of contents
- Cover Page
- Title Page
- Copyright Page
- Dedication
- Foreword by Alexzander A. A. Asea
- Foreword by Junfeng Ji
- Preface
- About the Authors
- Acknowledgments
- Contents
- Introduction
- Chapter 1 Lung Pattern Formation and Development
- Chapter 2 Advances in Lung Developmental Mechanobiology
- Chapter 3 Pulmonary Complications Associated with Preterm Birth
- Chapter 4 Lung Stem and Progenitor Cells
- Chapter 5 Pattern Formation of the Anterior Endoderm and Developing Lung
- Chapter 6 Lung Cell Polarity, Fate and Mode of Division
- Chapter 7 General Summary, Concluding Remarks and Future Directions
- References
- Index