
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Oxidation of C-H Bonds
Book details
Book preview
Table of contents
Citations
About This Book
A combination of oxidation methods and C?H bond functionalization, this book emphasizes mechanistic understanding and critical analysis of synthetic reactions to offer a guide or manual for practicing chemists. • Combines oxidation methods and C?H bond functionalization, two of the most important aspects of organic synthesis
• Deals with C?H bonds, an area of dynamic and continuous research across chemistry and catalysis
• Helps readers understand the fundamental and applied differences among various oxidation methods and reactions
• Covers mechanistic details, conditions, oxidation reagents, and practical aspects of different reactions
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Oxidation of C-H Bonds by Wenjun Lu, Lihong Zhou in PDF and/or ePUB format, as well as other popular books in Scienze fisiche & Chimica organica. We have over one million books available in our catalogue for you to explore.
Information
1
Introduction
1.1 What Is Oxidation of C─H Bonds?
Oxidation of C─H bonds is to transform the C─H bonds to various C─X bonds, in which X is a nonmetal atom with higher electronegativity than hydrogen, including carbon, nitrogen, oxygen, sulfur, selenium, fluorine, chlorine, bromine, iodine, etc. in this book [1]. In a typical oxidation process, it usually involves a cleavage of the covalent C─H bond and an oxidative functionalization of the carbon by a reagent (Scheme 1.1).
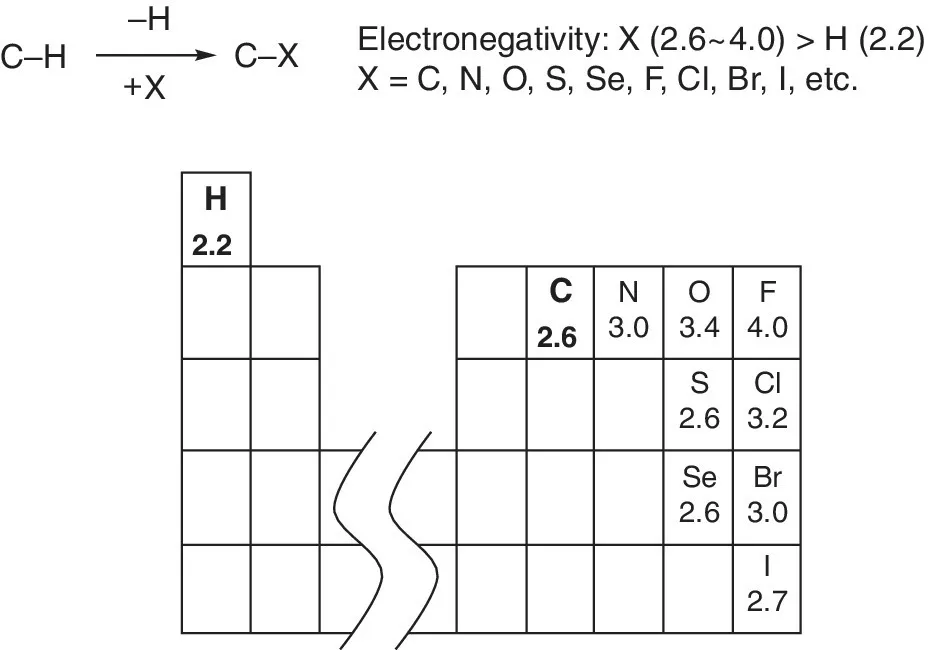
Scheme 1.1 Oxidation of C─H bond.
1.2 Chemical Synthesis and Oxidation of C─H Bonds
1.2.1 Transformation of Organic Compounds
Organic compounds are a kind of carbon molecules containing at least one C─H, C─C, or single C─heteroatom bond, which are very important substances to provide chemical energy, to construct organisms, to act as the functional materials in human life, and so on. Actually, many transformations are happening spontaneously among these organic compounds and other carbon‐containing compounds every day, leading to a big carbon cycle on the Earth. Meanwhile, man‐made organic compounds including agrochemicals, pharmaceuticals, and various organic functional materials are prepared enormously through a series of reactions from the raw materials such as methane, ethylene, and benzene, affecting the human being’s daily life and human beings themselves remarkably. The preparation of target products (complex molecules) from substrates (simple molecules) is called chemical synthesis normally involving multiple‐step reactions in one way (Scheme 1.2).
Scheme 1.2 Carbon cycle and chemical synthesis.
1.2.2 Ideal Chemical Synthesis
An ideal chemical synthesis is a process with minimal impact on external environment. There are two simple aspects in the process: mass and energy. In theory, at the end of the most ideal process, there are no other substances transformed except the desired products generated from substrates and no other energy consumed except the reaction heat ΔH for product generation. Although there is a large gap between the current chemical processes and the ideal ones in most cases, it is necessary to give some concise suggestions on the estimation of a practical process. Five rules for a HELLO process are listed as follows:
- High Yield When the product is obtained in high yield, it indicates that the utilization of substrate is highly sufficient and effective during the transformation.
- Efficient Pathway In an efficient pathway of chemical synthesis, a multiple‐step process is usually replaced by a one‐step reaction, a few reactions in one pot, or a cascade reaction to avoid or reduce the consumption of both substance and energy in the reactions and posttreatments. Furthermore, the substrates, intermediates, and products should be tolerant to the reaction systems without protection treatment on functional groups, and no external substances are consumed to initiate, accelerate, or control any reactions in the process.
- Low Loading If it is possible, to save substance, energy, and space, quantitative reactants are employed, and other necessary materials including catalysts, additives, and solvents are used at a minimum during the whole chemical process.
- Low Complexity in Operation It is highly required that a chemical process is carried out easily without special protection and caution, under normal pressure in air, and at an ambient temperature. In such a process, all expenses are reduced on safety, equipment, energy, and so on.
- Only Target Products In some cases, high selectivity such as high stereoselectivity is more important than high yield. Thus, it is the key symbol for an excellent and elegant process to obtain target products only. In other words, a HELLO process could become a HELL one with a poor selectivity because the wastes or by‐products generated could decrease obviously the quantity and/or quality of products and increase largely the cost of substance and energy in the reactions as well as in the product purifications.
To set up such a HELLO process, it mainly depends on the discovery and development of every single perfect reaction, that is, an ideal chemical synthesis is ...
Table of contents
- Cover
- Title Page
- Table of Contents
- Preface
- 1 Introduction
- 2 Oxidation of Methane
- 3 Oxidation of Alkyl sp3C─H Bond
- 4 Oxidation of Alkyl sp3C─H Bond Assisted by Directing Group
- 5 Oxidation of Alkyl sp3C—H Bond Adjacent to Unsaturated Carbon Atom
- 6 Oxidation of Alkyl sp3C─H Bond Adjacent to Heteroatom
- 7 Oxidation of Alkenyl or Carbonyl sp2C─H Bond
- 8 Oxidation of Alkynyl spC─H Bond
- 9 Oxidation of Benzene
- 10 Oxidation of Aryl sp2C─H Bond on Substituted Benzene
- 11 Oxidation of Aryl sp2C─H Bond Assisted by Directing Group
- 12 Oxidation of Aryl sp2C─H Bond on Heteroarene or Perfluoroarene
- 13 Oxidative Cross-Coupling of Aryl sp2C─H Bond with Inert C─H Bond
- Index
- End User License Agreement