
eBook - ePub
Arrow-Pushing in Organic Chemistry
An Easy Approach to Understanding Reaction Mechanisms
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Arrow-Pushing in Organic Chemistry
An Easy Approach to Understanding Reaction Mechanisms
Book details
Book preview
Table of contents
Citations
About This Book
Organic chemistry is required coursework for degrees in life, food, and medical sciences. To help the students discouraged by the belief that this topic cannot be mastered without significant memorization, Arrow Pushing in Organic Chemistry serves as a handy supplement for understanding the subject. • Includes new chapters, an expanded index, and additional problem sets complete with detailed solutions
• Focuses on understanding the mechanics and logic of organic reaction mechanisms
• Introduces ionic and non-ionic reactive species and reaction mechanisms
• Teaches strategies to predict reactive species, sites of reactions, and reaction products
• Provides a solid foundation upon which organic chemistry students can advance with confidence
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Arrow-Pushing in Organic Chemistry by Daniel E. Levy in PDF and/or ePUB format, as well as other popular books in Scienze fisiche & Chimica organica. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Introduction
The study of organic chemistry focuses on the chemistry of elements and materials essential for the existence of life. In addition to carbon, the most common elements present in organic molecules are hydrogen, oxygen, nitrogen, sulfur, and various halogens. Through the study of organic chemistry, our understanding of the forces binding these elements to one another and how these bonds can be manipulated are explored. In general, our ability to manipulate organic molecules is influenced by several factors that include the nature of functional groups near sites of reaction, the nature of reagents utilized in reactions, and the nature of potential leaving groups. In addition, these three factors impart further variables that influence the course of organic reactions. For example, the nature of the reagents used in given reactions can influence the reaction mechanisms and ultimately the reaction products. By recognizing the interplay between these factors and by applying principles of arrow‐pushing, which in reality represents bookkeeping of electrons, reasonable predictions of organic mechanisms and products can be realized without the burden of committing to memory the wealth of organic reactions studied in introductory courses. In this chapter, the concept of arrow‐pushing is defined in context with various reaction types, functional groups, mechanism types, reagents/nucleophiles, and leaving groups.
1.1 DEFINITION OF ARROW‐PUSHING
Organic chemistry is generally presented through a treatment of how organic chemicals are converted from starting materials to products. For example, the Wittig reaction (Scheme 1.1) is used for the conversion of aldehydes and ketones to olefins, and the Diels–Alder reaction (Scheme 1.2) is used for the formation of six‐membered ring systems and treatment of alkyl halides with reagents such as tributyltin hydride (Scheme 1.3), resulting in removal of the associated halides. However, by presenting these reactions as illustrated in Schemes 1.1, 1.2, and 1.3, no explanation is provided as to how the starting materials end up as their respective products.
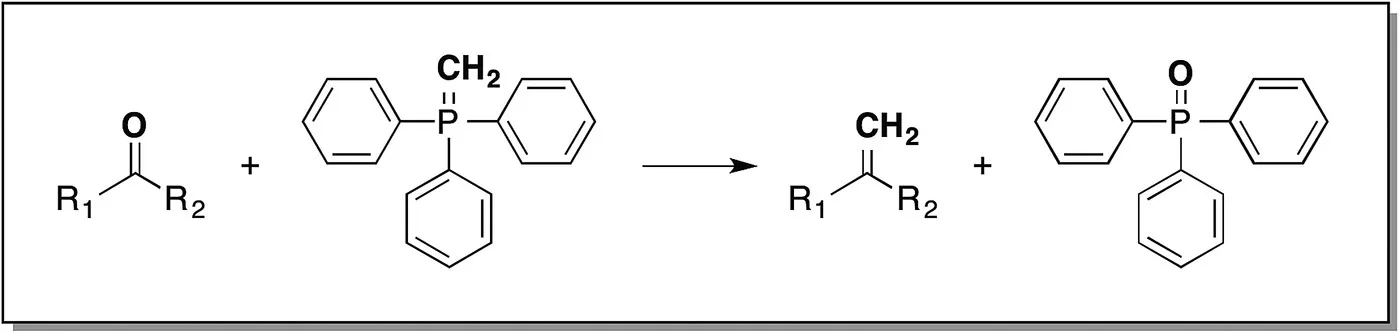
Scheme 1.1 Example of the Wittig reaction.
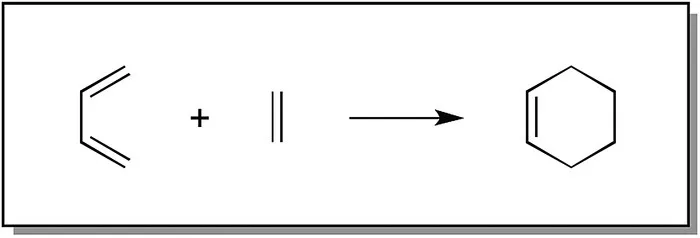
Scheme 1.2 Example of the Diels–Alder reaction.
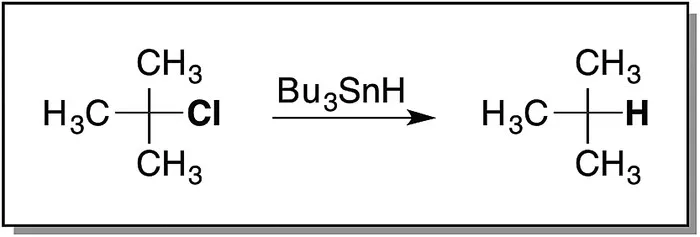
Scheme 1.3 Example of a tin hydride dehalogenation.
By definition, the outcome of any chemical reaction is the result of a process resulting in the breaking and formation of chemical bonds. Referring to material covered in most general chemistry courses, bonds between atoms are defined by sets of two electrons. Specifically, a single bond between two atoms is made of two electrons, a double bond between ato...
Table of contents
- Cover
- Title Page
- Table of Contents
- Preface
- Acknowledgements
- About the Author
- Chapter 1: Introduction
- Chapter 2: Free Radicals
- Chapter 3: Acids
- Chapter 4: Bases and Nucleophiles
- Chapter 5: SN2 Substitution Reactions
- Chapter 6: SN1 Substitution Reactions
- Chapter 7: Elimination Reactions
- Chapter 8: Addition Reactions
- Chapter 9: Carbenes
- Chapter 10: Pericyclic Reactions
- Chapter 11: Moving Forward
- Appendix 1: pKa Values of Protons Associated with Common Functional Groups
- Appendix 2: Answers and Explanations to Problems
- Appendix 3: Student Reaction Glossary
- Index
- Periodic Table of Elements
- End User License Agreement