
eBook - ePub
Application of IC-MS and IC-ICP-MS in Environmental Research
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Application of IC-MS and IC-ICP-MS in Environmental Research
Book details
Book preview
Table of contents
Citations
About This Book
Introduces the reader to the field of ion chromatography, species analysis and hyphenated methods IC-MS and IC-ICP-MS including the theory and theirs applications
- Covers the importance of species analysis and hyphenated methods in ion chromatography
- Includes practical applications of IC-MS and IC-ICP-MS in environmental analysis
- Details sample preparation methods for ion chromatography
- Discusses hyphenated methods IC-MS and IC-ICP-MS used in determining both the total element contents and its elements
- Details speciation analysis used in studying biochemical cycles of selected chemical compounds; determining toxicity and ecotoxicity of elements; food and pharmaceuticals quality control; and in technological process control and clinical analytics
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Application of IC-MS and IC-ICP-MS in Environmental Research by Rajmund Michalski, Rajmund Michalski in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Analytic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
CHAPTER 1
PRINCIPLES AND APPLICATIONS OF ION CHROMATOGRAPHY
Rajmund Michalski
Institute of Environmental Engineering, Polish Academy of Sciences, M. Skłodowskiej-Curie 34, 41-819, Zabrze, Poland
1.1 PRINCIPLES OF ION CHROMATOGRAPHY
1.1.1 Introduction
The history ofchromatography as a separation method began in 1903 when Mikhail Semyonovich Tsvet (a Russian biochemist working at the Department of Chemistry of the Warsaw University) separated plant dyes using adsorption in a column filled with calcium carbonate and other substances [1]. After extraction with the petroleum ether, he obtained clearly separated colorful zones. To describe this method, he used Greek words meaning color (ρωμα) and writing (γραϕω) and coined a new word, chromatography, which literally meant writing colors. At present, chromatographic methods are among the most popular instrumental methods in the analytical chemistry as they offer quick separation and determination of substances, including complex matrix samples.
Chromatographic methods are used widely on both the preparative and analytical scales. They help to separate and determine polar and nonpolar components; acidic, neutral, and alkaline compounds; organic and inorganic substances; monomers, oligomers, and polymers. It is necessary to use an appropriate chromatography type, which depends on the physicochemical properties of the examined sample and its components. Gas chromatography (GC) and liquid chromatography (LC) can be used to separate and determine approximately 20% and 80% of the known compounds, respectively. Ion chromatography (IC) is a part of high-performance liquid chromatography used to separate and determine anions and cations and also other substances after converting them into the ionic forms. In the literature, the term ion-exchange chromatography (I-EC) is found. It differs from ion chromatography even though both types are based on the widely known ion-exchange processes. Ion chromatography originates from ion-exchange chromatography. It uses high-performance analytical columns that are usually filled with homogenous particles with small diameters and most often conductometric detection. When compared to the classic ion-exchange chromatography, it is more efficient, faster, and more sensitive. It also offers very good repeatability of the obtained results. The ion-exchange chromatography term was used until 1975, when the first commercial ion chromatograph was available. At present, most analyses of ionic substances conducted with chromatographic techniques are performed with ion chromatography.
In the last 40 years, there were many state-of-the-art monographs that described the ion chromatography theory and applications in detail [2–5]. Some of these studies have already been republished. At present, there are three main separation methods in ion chromatography. They are based on different properties of substances used in the column phases and the resulting ion capacity. They include the following:
- Ion chromatography (IC) and can be either suppressed or nonsuppressed
- Ion exclusion chromatography (IEC)
- Ion pair chromatography (IPC).
The block diagram of an ion chromatograph (cation-exchange and anion-exchange types), together with ion-exchange reactions for the most popular suppressed ion chromatography, can be seen in Figure 1.1.
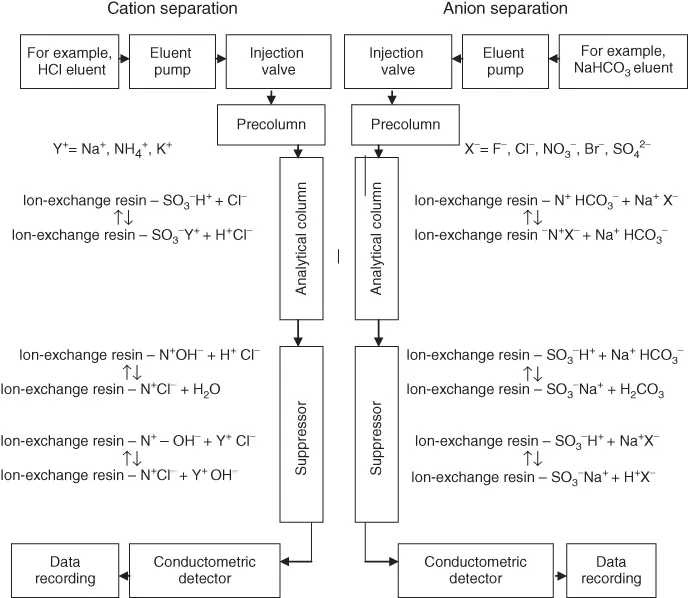
Figure 1.1 Block diagram of an ion chromatograph with a conductometric detector.
The anion separation proceeds according to the following principle: analyte ions (e.g., Cl−) together with eluent ions pass through the analytical column in which the following ion-exchange reaction takes place:
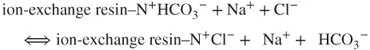
The affinity of the analyte ions toward the stationary phase is diverse. Consequently, the ions are separated and leached out from the analytical column within different retention times against the background of weakly dissociated NaHCO3. Afterward, they are transported into the suppressor with high-capacity sulfonic cation exchanger. The following reaction takes place:
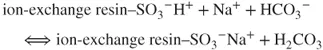
The NaHCO3 eluent ions are transformed into weakly dissociated carbonic acid due to the occurring reactions. The analyte ions (e.g., Cl−) react in accordance with the following formula:
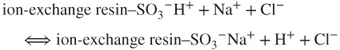
Due to the reactions taking place in the analytical column and the suppressor, the analyte ions reach the detector in the form of strongly dissociated acids against the background of weakly dissociated carbonic acid. The obtained signal related to the conductivity of the analyte ions (the analyte forms a well-dissociated salt after the reactions) is high enough to use the conductometric detector to record the peaks of separated anions against the background of a weak signal related to the low eluent conductivity (forming weakly dissociated carbonic acid). Parallel reactions are observed when cations are determined. The cation-exchange column is filled with a cation exchanger with sulfonic groups. Eluent consists of water solution of, for example, hydrochloric acid. The analyte ions (e.g., Na+) together with the eluent ions pass through the analytical column in which the following ion-exchange reaction takes place:
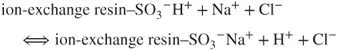
The affinity of the analyte ions toward the stationary phase is diverse. Consequently, the cations are separated and leached out from the analytical column within different retention times against the background of strongly dissociated HCl. Afterward, the ions are transported into the suppressor with high-capacity anion exchanger (e.g., with quaternary ammonium groups as functional groups). The following chemical reaction occurs:
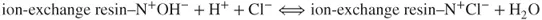
The HCl eluent ions are transformed into water due to the reactions in the suppressor, whereas the analyte ions (Na+) react with the exchanger in the suppression column according to the following formula:
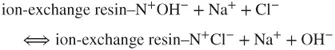
Due to the chemical reactions in the analytical column and suppressor, the analyte ions reach the detector in the form of highly dissociated hydroxides against the water background, which allows analysis in the conductometric detector.
Ion exclusion chromatography (IEC) is a comparatively old technique, which uses the Gibbs–Donnan effect. A porous ionic-exchanger functions as a semipermeable membrane separating two water phases (mobile and stationary) contained in the exchanger pores. The membrane is only permeable for nonionized or weakly ionized substances. They are separated between two water phases, wherea...
Table of contents
- COVER
- TITLE PAGE
- COPYRIGHT
- TABLE OF CONTENTS
- LIST OF CONTRIBUTORS
- PREFACE
- CHAPTER 1: PRINCIPLES AND APPLICATIONS OF ION CHROMATOGRAPHY
- CHAPTER 2: MASS SPECTROMETRIC DETECTORS FOR ENVIRONMENTAL STUDIES
- CHAPTER 3: HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY COUPLED TO INDUCTIVELY COUPLED PLASMA MS/ELECTROSPRAY IONIZATION MS
- CHAPTER 4: APPLICATION OF IC-MS IN ORGANIC ENVIRONMENTAL GEOCHEMISTRY
- CHAPTER 5: ANALYSIS OF OXYHALIDES AND HALOACETIC ACIDS IN DRINKING WATER USING IC-MS AND IC-ICP-MS
- CHAPTER 6: ANALYSIS OF VARIOUS ANIONIC METABOLITES IN PLANT AND ANIMAL MATERIAL BY IC-MS
- CHAPTER 7: ANALYSIS OF PERCHLORATE ION IN VARIOUS MATRICES USING ION CHROMATOGRAPHY HYPHENATED WITH MASS SPECTROMETRY
- CHAPTER 8: SAMPLE PREPARATION TECHNIQUES FOR ION CHROMATOGRAPHY
- INDEX
- END USER LICENSE AGREEMENT