
Green Oxidation in Organic Synthesis
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Green Oxidation in Organic Synthesis
About This Book
A valuable introduction to green oxidation for organic chemists interested in discovering new strategies and new reactions for oxidative synthesis
Green Oxidation in Organic Synthesis provides a comprehensive introduction and overview of chemical preparation by green oxidative processes, an entry point to the growing journal literature on green oxidation in organic synthesis. It discusses both experimental and theoretical approaches for the study of new catalysts and methods for catalytic oxidation and selective oxidation.
The book highlights the discovery of new reactions and catalysts in recent years, discussing mechanistic insights into the green oxidative processes, as well as applications in organic synthesis with significant potential to have a major impact in academia and industry. Chapters are organized according to the functional groups generated in the reactions, presenting interesting achievements for functional group formation by green oxidative processes with O 2, H 2 O 2, photocatalytic oxidation, electrochemical oxidation, and enzymatic oxidation. The mechanisms of these novel transformations clearly illustrated.
Green Oxidation in Organic Synthesis will serve as an excellent reference for organic chemists interested in discovering new strategies for oxidative synthesis which address the priorities of green and sustainable chemistry.
Frequently asked questions
1
Green Oxidative: Synthesis of Alcohols and Phenols
1.1 Introduction
1.2 C─H Hydroxylation via Aerobic Oxidant
1.2.1 Synthesis of Phenols by C(sp2)‐H Hydroxylation
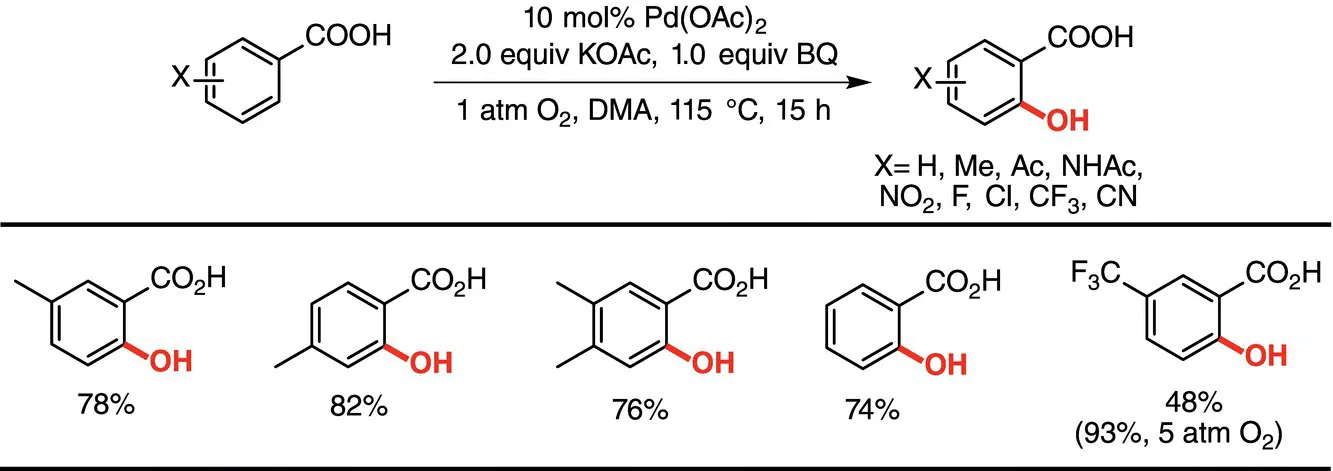
Table of contents
- Cover
- Table of Contents
- Preface
- List of Contributors
- 1 Green Oxidative
- 2 Green Oxidative Synthesis of Aldehydes and Ketones
- 3 Green Oxidative Synthesis of Ethers, Esters, and Organic Halides
- 4 Green Oxidative Synthesis of Epoxides
- 5 Green Oxidative Synthesis of Carboxylic Acids
- 6 Green Oxidative Synthesis of Amines, Amides, and Imines
- 7 Green Oxidative Synthesis of Nitriles
- 8 Green Oxidative Synthesis of Azo, Diazo, and Azido Compounds
- 9 Green Oxidative Synthesis of Substituted Olefins and Alkynes
- 10 Green Oxidative Synthesis of Substituted Arenes
- 11 Green Oxidative Synthesis of Heterocyclic Compounds
- 12 Green Oxidation of Sulfide to Sulfoxide and Sulfone
- 13 Oxidative Couplings with C─H Bonds Forming C─P and C─S Bonds
- 14 The Recent Developments of Photocatalytic Oxidation
- 15 The Recent Developments of Electrochemical Oxidation
- 16 The Recent Developments of Enzymatic Oxidation
- Index
- End User License Agreement