
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Book details
Book preview
Table of contents
Citations
About This Book
Written by highly renowned and experienced authors, this is the only reference on the application of solvents as reagents.
Clearly structured, the text describes various methods for the activation and reaction of these small molecules, highlighting the synthetic opportunities as well as process-oriented advantages. To this end, all relevant types of solvents are covered separately and emphasized with numerous synthetic examples, while taking care to explain applications so as to avoid undesired side reactions.
The result is a unique resource for every synthetic chemist and reaction engineer in industry and academia working on the methodical optimization of synthetic transformations.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Solvents as Reagents in Organic Synthesis by Xiao-Feng Wu in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
The Applications of Water as Reagents in Organic Synthesis
Zhengkai Chen and Hongjun Ren
Zhejiang Sci-Tech University, Department of Chemistry, Hangzhou, 310018, PR China
1.1 Introduction
Water due to its low cost, easy availability, nontoxic and nonflammable properties has been considered one of the most ideal and promising solvents in organic synthesis from the green and sustainable point of view. Furthermore, with regard to enormous enzyme-catalyzed biosynthesis in nature, water serves as a favorable medium for the versatile synthesis of a variety of complicated molecules and compounds. Over the past decades, considerable efforts had been devoted into the organic reactions by using water as solvent from economy and environment perspectives [1]. Even more, the proposed concept of “in-water” and “on-water” further stimulated the booming development of the utilization of water as solvent for organic synthesis [1e, 2]. Therefore, in recent years, more and more general organic reactions were successfully exploited to perform in water instead of organic solvents to achieve sustainable and environmental benefits.
From a different perspective, the water itself could also be applied as a useful reagent to participate in the reaction through incorporating a hydrogen or oxygen atom or hydroxyl group into the target product. Generally, water is indispensable for various hydrolysis reactions. As a hydrogen source, water is used to quench numerous susceptible reaction systems by providing active hydrogen. Meanwhile, as a versatile nucleophile, the hydroxyl group could be readily introduced into the specific reaction sites by the employment of water as hydroxyl precursor. The hydroxyl group could also be readily oxidized to carbonyl group during the reaction. It is worth mentioning that, in some cases, the presence of water could obviously improve the efficiency of the reaction, albeit the exact reason is elusive for some special reactions.
This chapter is divided into the following four parts for further discussion: (i) incorporation of hydrogen atom from water; (ii) incorporation of oxygen atom from water; (iii) incorporation of hydroxyl group from water; and (iv) traceless promotion of the reactions by water.
1.2 Incorporation of Hydrogen Atom from the Water
Aggarwal and coworkers demonstrated a versatile strategy through lithiation/borylation/protodeboronation of a homoallyl carbamate for the highly enantioselective synthesis of (+)-sertraline and (+)-indatraline, which served as potent inhibitors (Scheme 1.1) [3]. It was observed that the presence of the alkene could hamper the lithiation/borylation process, so the modifications of the reaction conditions were necessary by the use of 12-crown-4, TMSCl, H2O or a solvent switch to achieve 1,2-metalate rearrangement in order to ensure high yields and enantioselectivity. As for the protodeboronation step of tertiary boronic ester, the amount of water played a crucial role in the reaction. In 2010, the same group had disclosed a simple approach for the protodeboronation of tertiary boronic esters employing CsF-H2O or TBAF·3H2O with complete stereoselectivity to access to diverse enantioenriched tertiary alkanes [4].
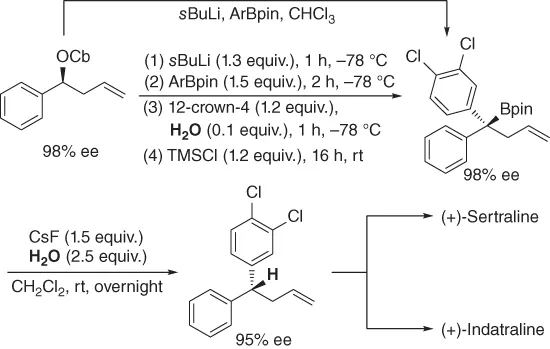
Scheme 1.1 Enantioselective synthesis of (+)-sertraline and (+)-indatraline.
A catalyst-free sulfonylation of activated alkenes with sulfonyl hydrazides in water for highly efficient construction of monosubstituted ethyl sulfones was demonstrated by Wang and coworkers (Scheme 1.2) [5]. Remarkably, the reaction proceeded through without any catalyst, additive, ligand, or organic solvent, with the release of N2 as single by-product. The results of control experiments indicated that an anion pathway was involved in the reaction and the α-hydrogen atom of β-sulfone esters originated from water.
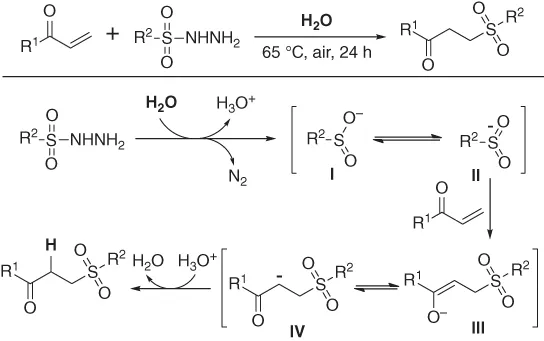
Scheme 1.2 Catalyst-free sulfonylation of activated alkenes in water.
The sulfinyl anion I was first formed assisted by water with the release of one molecule of N2, which could transform into sulfur-centered anion II through resonance process in the presence of water. The sulfur-centered anion readily added to the activated alkene to give the oxygen-centered anion III, followed by another resonance interaction leading to the carbon-centered anion IV. Finally, the proton transfer of intermediate IV from hydronium ions to deliver the desired β-sulfone ester product.
A silver(I)-catalyzed chemo- and regioselective hydroazidation of ethynyl carbinols for the construction of 2-azidoallyl alcohols was developed by Bi and coworkers (Scheme 1.3) [6]. In this transformation, trimethylsilyl azide (TMS-N3) was chosen as the optimal azide source and the pendent hydroxy...
Table of contents
- Cover
- Title Page
- Copyright
- Table of Contents
- List of Contributors
- Chapter 1: The Applications of Water as Reagents in Organic Synthesis
- Chapter 2: The Applications of Toluene and Xylenes
- Chapter 3: The Applications of 1,4-Dioxane, THF, and Ethers as Versatile Building Blocks in Organic Synthesis
- Chapter 4: The Application of Dichloromethane and Chloroform as Reagents in Organic Synthesis
- Chapter 5: The Applications of Acetone and Ethyl Acetate
- Chapter 6: N,N-Dimethylformamide and N,N-Dimethylacetamide as Carbon, Hydrogen, Nitrogen, and/or Oxygen Sources
- Chapter 7: The Applications of DMSO
- Chapter 8: Acetonitrile as Reagents in Organic Synthesis: Reactions and Applications
- Chapter 9: The Applications of Nitromethane as Reagent and Solvent in Organic Synthesis
- Chapter 10: Alcohol as a Reagent in Homogeneous Catalysis
- Chapter 11: Synchronous Application of Hydrocarbons as Solvents and Reagents in Transition-Metal Catalysis
- Index
- End User License Agreement