
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Hydrogen Bonding in Polymeric Materials
About this book
Summarizing our current knowledge of the topic, this book describes the roles and effects of hydrogen bonding in polymer materials by reviewing the latest developments over recent years.
To this end, it discusses all relevant aspects from the fundamentals, via characterization, to properties and applications in various polymeric materials, including polymer blends, block copolymers, mesoporous materials, biomacromolecules and nanocomposites.
Invaluable reading for scientists in polymers and materials as well as those working in macromolecular chemistry.
To this end, it discusses all relevant aspects from the fundamentals, via characterization, to properties and applications in various polymeric materials, including polymer blends, block copolymers, mesoporous materials, biomacromolecules and nanocomposites.
Invaluable reading for scientists in polymers and materials as well as those working in macromolecular chemistry.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Hydrogen Bonding in Polymeric Materials
1.1 Introduction
Hydrogen-bonding, dipole–dipole, and ionic interactions in polymers have been of great interest to fundamental polymer science, and also industrially, for over 30 years. These secondary or noncovalent interactions can be introduced specifically into polymeric materials to form supramolecular materials displaying interesting thermal, mechanical, surface, and optoelectronic properties. The concept of noncovalent bonding has changed the thinking of polymer scientists, who had been focused for many years primarily on the effects of covalent interactions.
Hydrogen bonds (H-bonds) are interactions that result from dipole–dipole forces between strongly electronegative atoms (e.g., fluorine (F), nitrogen (N), oxygen (O)), and hydrogen atoms; they affect the physical properties and microstructures of many materials [1–6]. For example, water is recognized to form tetrahedral clusters comprising 14 molecules of H2O; the unusual properties of water arise mainly from the fact that water molecules readily form H-bonds—4 of them—per water molecule, in a tetrahedral geometry [7]. Other famous examples are the H-bonds found in biological systems [8], where they play important roles affecting the three-dimensional structures of nucleic bases and proteins. The DNA double helix is formed from multiple H-bonding interactions between complementary cytosine/guanine (C/G) and adenine/thymine (A/T) base pairs; these noncovalent interactions link the two complementary strands and enable replication. Furthermore, H-bonds greatly influence the secondary structures of polypeptides: the α-helix conformation is stabilized by intramolecular (or intrachain) H-bonding, while the β-sheet conformation is stabilized by intermolecular (or interchain) H-bonding [9, 10]. In addition to these famous natural examples, H-bonding also has several profound effects in unnatural polymeric materials, influencing various physical, thermal, and mechanical properties, including melting points (Tm), crystalline structures, glass transition temperatures (Tg), surface properties, optoelectronic properties, and solubilities (in solvents) and miscibilities (in polymer blends).
Although there are already several reviews on H-bonded polymer blends, copolymers, self-assembled supramolecular structures, and nanocomposite systems [11–21], this book aims to provide a thorough discussion of how H-bonding interactions have been used in research into polymer blends, surface properties, self-assembled block copolymers, mesoporous materials, biomacromolecules, and polyhedral oligomeric silsesquioxanes (POSS) nanocomposites.
1.1.1 Hydrogen Bonds
Hydrogen bonding is a fundamental interaction in chemistry, physics, and biology and has been described extensively in many books [22] and reviews [23]. The H-bond is a directed, attractive, noncovalent bonding interaction between an A–H unit (proton donor) and a B atom (proton acceptor) in the same molecule or in different molecules, where the A and B atoms are generally highly electronegative (e.g., F, N, O), although even C–H groups can be involved in H-bonding and some π-electrons can act as weak H-bond acceptors [24, 25]. The H-bond can also be characterized by its effect on the physical properties or molecular characteristics of a material. A covalent bond usually has strength on the order of 50 kcal mol−1; H-bonds most often have stabilities in the range 1–40 kcal mol−1 (in comparison, van der Waals attraction is favorable by only approximately 0.2 kcal mol−1). A strong H-bond has strength in the range 10–40 kcal mol−1; a moderate H-bond, 4–10 kcal mol−1; and a weak H-bond, 1–4 kcal mol−1 [26].
Hydrogen bonding can be either an intermolecular or intramolecular phenomenon. An intermolecular H-bond (Figure 1.1) is one for which the donor and acceptor units are found in two different molecules; for an intramolecular H-bond (Figure 1.2) they are in the same molecule. Intermolecular H-bonds are usually linear or near linear, whereas intramolecular H-bonds usually feature some degree of bending. In polymers, two different types of H-bonding can occur for the same functional group, namely, interchain and intrachain H-bonding interactions. For example, the α-helix conformation of a polypeptide is stabilized by intrachain H-bonding, while the β-sheet conformation is stabilized by interchain H-bonding. The strength of an H-bond is strongly dependent on the solvent polarity; the addition of a polar solvent can decrease the H-bond strength significantly, over several orders of magnitude, because the solvent molecule can also take part in H-bonding interactions. As a result, nonpolar solvents (e.g., toluene, CHCl3, and linear and cyclic alkanes) are mostly used for the preparation of H-bonded supramolecular materials.
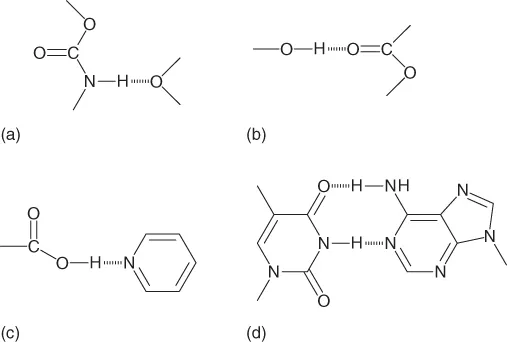
Figure 1.1 Intermolecular H-bonding between two molecules. (a) Urethane–ether complex; (b) hydroxyl–carbonyl complex; (c) acid–pyridine complex; and (d) adenine–thymine complex.
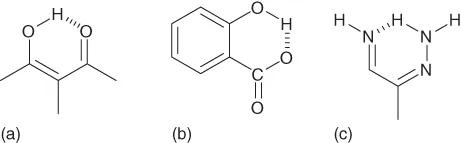
Figure 1.2 Intramolecular H-bonding of a single molecule. (a) Malonaldehyde; (b) salicyclic acid; and (c) formazan.
1.1.2 Characterization of Hydrogen Bonding
Several spectroscopic methods are commonly used to characterize H-bonds: (i) Fourier transform infrared (FTIR) and Raman spectroscopy, in which the stretching and bending vibrations of the donor or acceptor functional groups are influenced by the presence of H-bonds; (ii) ultraviolet (UV) and fluorescence spectroscopy, which reveal changes in the electronic levels of molecules experiencing H-bond interactions; (iii) nuclear magnetic resonance (NMR) spectroscopy, where changes in chemical shifts can arise from H-bond interactions of the donor and acceptor functional groups; and (iv) X-ray photoelectron (XPS) spectroscopy, where a new shoulder or even a new peak can appear as a result of a change in the chemical environment of an atom perturbed by the H-bonding [27–30].
Among these methods for characterizing H-bonds, by far the most inexpensive and sensitive is FTIR spectroscopy. For example, Figure 1.3 presents the CO stretching range of the FTIR spectra of H-bonded phenolic/PCL blends of various compositions. The signal for C=O stretching in this phenolic/PCL blend splits into two bands: a signal at higher wavenumber (1734 cm–1) correspo...
Table of contents
- Cover
- Title Page
- Copyright
- Table of Contents
- Preface
- Abbreviation
- Chapter 1: Hydrogen Bonding in Polymeric Materials
- Chapter 2: Hydrogen Bonding in Polymer Blends
- Chapter 3: Physical Properties of Hydrogen-Bonded Polymers
- Chapter 4: Surface Properties of Hydrogen-Bonded Polymers
- Chapter 5: Sequence Distribution Effects in Hydrogen-Bonded Copolymers
- Chapter 6: Hydrogen Bond-Mediated Self-Assembled Structures of Block Copolymers
- Chapter 7: Mesoporous Materials Prepared Through Hydrogen Bonding
- Chapter 8: Bioinspired Hydrogen Bonding in Biomacromolecules
- Chapter 9: Hydrogen Bonding in POSS Nanocomposites
- Index
- End User License Agreement
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Hydrogen Bonding in Polymeric Materials by Shiao-Wei Kuo in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physics. We have over one million books available in our catalogue for you to explore.