eBook - ePub
Preparative Chromatography for Separation of Proteins
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Preparative Chromatography for Separation of Proteins
Book details
Book preview
Table of contents
Citations
About This Book
Preparative Chromatography for Separation of Proteins addresses a wide range of modeling, techniques, strategies, and case studies of industrial separation of proteins and peptides. • Covers broad aspects of preparative chromatography with a unique combination of academic and industrial perspectives
• Presents Combines modeling with compliantce useing of Quality-by-Design (QbD) approaches including modeling
• Features a variety of chromatographic case studies not readily accessible to the general public
• Represents an essential reference resource for academic, industrial, and pharmaceutical researchers
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Preparative Chromatography for Separation of Proteins by Arne Staby, Anurag S. Rathore, Satinder Ahuja, Arne Staby, Anurag S. Rathore, Satinder Ahuja in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Chemical & Biochemical Engineering. We have over one million books available in our catalogue for you to explore.
Information
1
Model‐Based Preparative Chromatography Process Development in the QbD Paradigm
Arne Staby1, Satinder Ahuja2, and Anurag S. Rathore3
1 CMC Project Planning & Management, Novo Nordisk A/S, Bagsværd, Denmark
2 Ahuja Consulting, Calabash, NC, USA
3 Department of Chemical Engineering, Indian Institute of Technology, New Delhi, India
1.1 Motivation
Preparative chromatography for separation of proteins and peptides continues to be the primary workhorse in purification of biopharmaceuticals. Numerous papers and books exist describing theory and implementation of preparative chromatography; however, this is the first book that combines academic progress in modeling with industrial implementation. Although theory and models have been available for many years, industrial usage of these tools has been scarce due to labor‐ and material‐intensive requirements. However, with the biotech industry moving to implement the expectations underlined in the recent regulatory initiative of quality by design (QbD), interesting and outspread applications of modeling tools for commercial process development and manufacture have emerged.
1.2 Regulatory Context of Preparative Chromatography and Process Understanding
QbD expectations to biopharmaceutical production including preparative chromatography are described in the ICH quality guidelines Q8, Q9, Q10, and Q11 [1–4]. Further, ICH Q8‐R2 [1] provides the overall definition of QbD in a regulatory context.
A systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control based on sound science and quality risk management.
The focus of this book is on the underlined parts of this definition, and the framework of QbD may be outlined as presented in Figure 1.1. In the top part of the figure, the primary focus of biopharmaceuticals is the patient, and the patient needs are defined through the quality target product profile (QTPP), which in turn is affected by chemistry, manufacturing, and controls (CMC) activities. Fulfilling patients’ needs places some requirements on the product, and these elements are obtained through linkage of the QTPP to the list of critical quality attributes (CQAs). The CQAs will have acceptable ranges for the manufacturer to comply with, and to obtain product of the desired quality, the process needs to be run within acceptable ranges of process parameters. Proper knowledge of how process parameters affect the product quality may be obtained through process models that may end up in a regulatory, enhanced application for approval of a design space. To control process parameters within defined ranges, process models and/or even a design space will provide some requirements to the GMP facility and linkage to the control strategy, which will include various process monitors, process analytical technology (PAT) tools, process validation, and release tests and specifications. All elements are linked through risk assessment exercises to address the risk‐based approach of QbD in a regulatory setting.
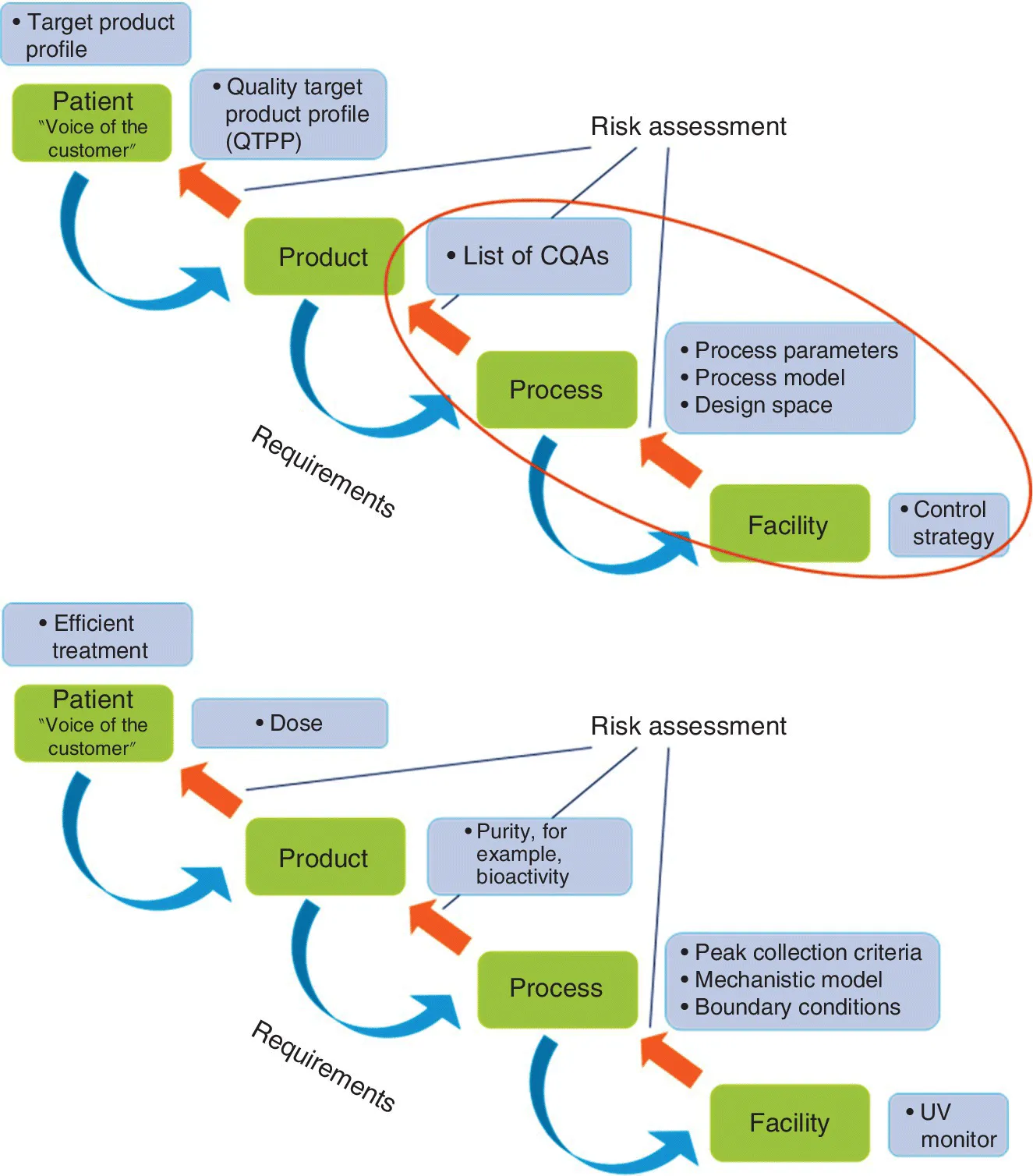
Figure 1.1 (Top) The framework of QbD. (Bottom) Example of QbD elements contained in the QbD framework for a preparative chromatography step.
Figure 1.1 (bottom) displays an example of QbD elements contained in the QbD framework for a preparative chromatography step. A key patient need is of course to get efficient treatment, and one element affecting this is to get a proper dose of the biopharmaceutical. To obtain proper dosing, the purity and among others the bioactivity of the biopharmaceutical needs to be correct. Purity is significantly affected by the peak collection criteria used in preparative chromatography, and a well‐known methodology for peak collection is by UV monitoring as part of the control strategy (e.g., see Chapters 12 and 17). A proper understanding and control of the preparative chromatography process may be obtained by a mechanistic or statistical model and their boundary conditions that may define an operational design space. Thus, the idea of this linkage exercise is to obtain a complete overview of the process in a way that will elucidate, for example, how a defect in or removal of a UV monitor in a preparative chromatographic purification step will affect the patient through cascading back in the figure through a series of risk assessments. The focus of this book is to obtain “process understanding and process control based on sound science” as described earlier, and it can be visualized by observing the elements within the red circle in Figure 1.1 (top).
A proper control strategy is achieved through sufficient process understanding. Traditionally, process understanding in the biopharmaceutical industry was obtained through a combination of theoretical knowledge based on the following: (i) education; (ii) experience from other projects and proteins optionally of similar nature, for example, mAbs; (iii) preliminary experimentation of less systematic nature; and (iv) “one parameter at a time” (OPAT) experimentation where all variables are kept constant while systematically altering one variable. This concept has worked well for many years, and most legacy products have been developed using this approach. Figure 1.2 presents the general level of knowledge obtained by the different methodologies including more recent concepts. Although some companies have also used multivariate methods for development and documentation of legacy products, the extensive use of more advanced methods for process understanding has been affected by implementation of QbD concepts. The general methodology used in the industry today is based on multivariate statistical analysis such as design of experiments (DoE) often combined with various high‐throughput process development (HTPD) techniques (see e.g., Chapter 11). DoE is a very broad and important tool that does not require mechanistic understanding prior to implementation, and it works quite efficiently if the user has prior knowledge of which parameters are significant and if the number of parameters is limited. Today, the most comprehensive application of statistical methods to support QbD and a true enhanced approach filing has been accomplished by Genentech/Roche with its recent regulatory approval of Gazyva. Disadvantages of DoE include less optimal identification of assumptions and the general lack of opportunities for extrapolation outside the experimental area used to set up the statistical models. DoE is used extensively for validation of parameter ranges in preparative chromatography; however for other unit operations such as fermentation, more advanced statistical methods like principal component analysis (PCA), partial least squares (PLS) methods, etc. are used due to their capability to handle very high number of variables (see also Chapter 16). At the top of the pyramid in Figure 1.2 and at the highest extent of knowledge obtainable are models based on mechanistic principles because full mechanistic process understanding is typically achieved. Depending on assumptions, these mechanistic models are also referred to as first‐principle models, and they provide...
Table of contents
- Cover
- Title Page
- Table of Contents
- List of Contributors
- Series Preface
- Preface
- 1 Model‐Based Preparative Chromatography Process Development in the QbD Paradigm
- 2 Adsorption Isotherms
- 3 Simulation of Process Chromatography
- 4 Simplified Methods Based on Mechanistic Models for Understanding and Designing Chromatography Processes for Proteins and Other Biological Products‐Yamamoto Models and Yamamoto Approach
- 5 Development of Continuous Capture Steps in Bioprocess Applications
- 6 Computational Modeling in Bioprocess Development
- 7 Chromatographic Scale‐Up on a Volume Basis
- 8 Scaling Up Industrial Protein Chromatography
- 9 High‐Throughput Process Development
- 10 High‐Throughput Column Chromatography Performed on Liquid Handling Stations
- 11 Lab‐Scale Development of Chromatography Processes
- 12 Problem Solving by Using Modeling
- 13 Modeling Preparative Cation Exchange Chromatography of Monoclonal Antibodies
- 14 Model‐Based Process Development in the Biopharmaceutical Industry
- 15 Dynamic Simulations as a Predictive Model for a Multicolumn Chromatography Separation
- 16 Chemometrics Applications in Process Chromatography
- 17 Mid‐UV Protein Absorption Spectra and Partial Least Squares Regression as Screening and PAT Tool
- 18 Recent Progress Toward More Sustainable Biomanufacturing
- Index
- End User License Agreement