
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Clinical Investigations at a Glance
About This Book
Clinical Investigations at a Glance
The market-leading at a Glance series is popular among healthcare students and newly qualified practitioners, for its concise and simple approach and excellent illustrations.
Each bite-sized chapter is covered in a double-page spread with clear, easy-to-follow diagrams, supported by succinct explanatory text.
Covering a wide range of topics, books in the at a Glance series are ideal as introductory texts for teaching, learning and revision, and are useful throughout university and beyond.
Everything you need to know about Clinical Investigations... at a Glance!
Clinical Investigations at a Glance provides an up-to-date, evidence-based overview of diagnostic investigations, looking at their choice, importance and interpretation for commonly presenting symptoms and conditions.
Designed to help develop the evidence-based use of investigations and interpret results properly, the book provides a unique perspective on many critical issues in medical testing, with the aim of improving diagnostic accuracy and reducing unnecessary tests or harm.
Clinical Investigations at a Glance is structured in three parts: an overview of tests; common presentations (such as chest pain, nausea and vomiting, weight loss and anaemia); and conditions organized by body system, such as cardiovascular disease, respiratory disease and nephrology.
Key features include:
- How to interpret investigations, using high quality illustrations to compare 'normal' and 'diseased' results
- Evidence-based, including references
- How to select the most appropriate investigation, the accuracy of tests and how to manage incidental findings
For more information on the complete range of Wiley medical student and junior doctor publishing, please visit: www.wileymedicaleducation.com
To receive automatic updates on Wiley books and journals, join our email list. Sign up today at www.wiley.com/email
All content reviewed by students for students
Wiley Medical Education books are designed exactly for their intended audience. All of our books are developed in collaboration with students. This means that our books are always published with you, the student, in mind.
If you would like to be one of our student reviewers, go to www.reviewmedicalbooks.com to find out more.
This title is also available as an e-book. For more details, please see www.wiIey.com/buy/9781118759325
Frequently asked questions
Information
Part 1 Overview of tests
Chapters
- 1 Investigations and interpreting tests
- 2 Screening tests
- 3 Consent for investigations
- 4 Risks of tests
- 5 Genetic tests
- 6 Principles of radiological investigations
- 7 Cytology and histopathology tests
- 8 Presenting at radiology and histopathology meetings
- 9 Requesting tests
- 10 Therapeutic drug monitoring
- 11 Investigations in resource-limited settings
- 12 Tests for outpatient, inpatient and patients in intensive care
1 Investigations and interpreting tests
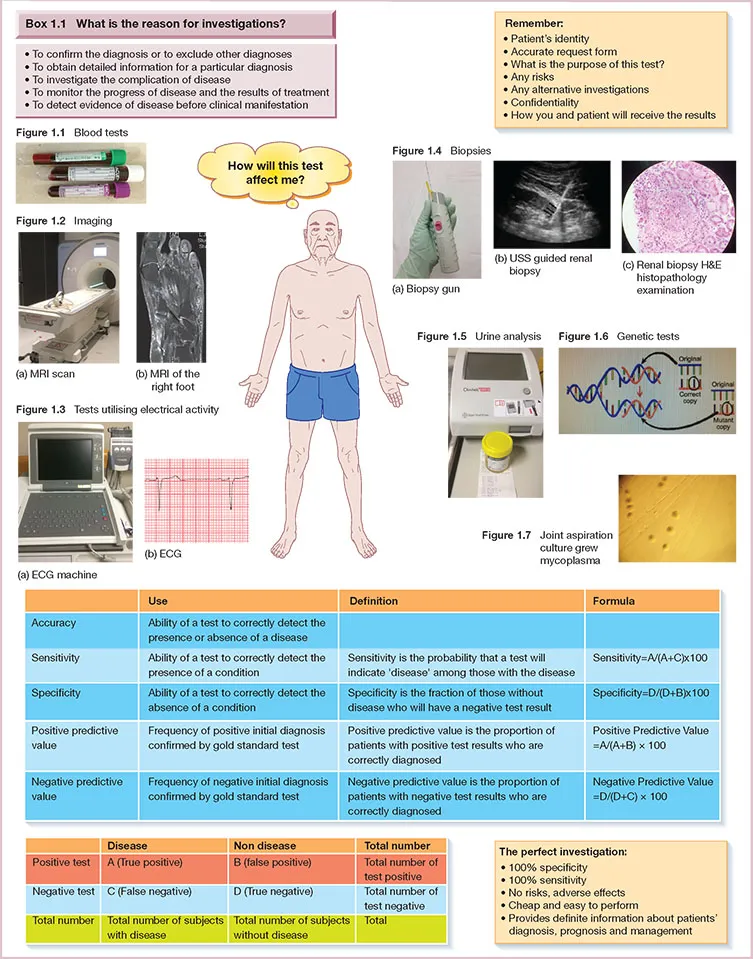
The result
Confidentiality
Repeating tests
Specificity and sensitivity
Receiver operator characteristic curves
Measures of disease probability

Normal range
Incidental findings: none of us are ‘normal’
Table of contents
- Cover
- Dedication
- Title Page
- Copyright
- Preface
- Acknowledgements
- Abbreviations
- Part 1: Overview of tests
- Part 2: Common presentations
- Part 3: Conditions
- Further reading
- Index
- EULA