
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About This Book
This is the first scientic book devoted to the Pauli exclusion principle, which is a fundamental principle of quantum mechanics and is permanently applied in chemistry, physics, and molecular biology. However, while the principle has been studied for more than 90 years, rigorous theoretical foundations still have not been established and many unsolved problems remain.
Following a historical survey in Chapter 1, the book discusses the still unresolved questions around this fundamental principle. For instance, why, according to the Pauli exclusion principle, are only symmetric and antisymmetric permutation symmetries for identical particles realized, while the Schrödinger equation is satisfied by functions with any permutation symmetry? Chapter 3 covers possible answers to this question. The construction of function with a given permutation symmetry is described in the previous Chapter 2, while Chapter 4 presents effective and elegant methods for finding the Pauli-allowed states in atomic, molecular, and nuclear spectroscopy. Chapter 5 discusses parastatistics and fractional statistics, demonstrating that the quasiparticles in a periodical lattice, including excitons and magnons, are obeying modified parafermi statistics.
With detailed appendices, The Pauli Exclusion Principle: Origin, Verifications, and Applications is intended as a self-sufficient guide for graduate students and academic researchers in the fields of chemistry, physics, molecular biology and applied mathematics. It will be a valuable resource for any reader interested in the foundations of quantum mechanics and its applications, including areas such as atomic and molecular spectroscopy, spintronics, theoretical chemistry, and applied fields of quantum information.
Frequently asked questions
Information
1
Historical Survey
1.1 Discovery of the Pauli Exclusion Principle and Early Developments
The anomalous type of splitting was especially fruitful because it exhibited beautiful and simple laws, but on the other hand it was hardly understandable, since very general assumptions concerning the electron using classical theory, as well as quantum theory, always led to the same triplet. A closer investigation of this problem left me with the feeling, it was even more unapproachable. A colleague who met me strolling rather aimlessly in the beautiful streets of Copenhagen said to me in a friendly manner, ‘You look very unhappy’; whereupon I answered fiercely, ‘How can one look happy when he is thinking about the anomalous Zeeman effect?’
According to this point of view, the doublet structure of alkali spectra … is due to a particular two‐valuedness of the quantum theoretic properties of the electron, which cannot be described from the classical point of view.
In an atom there cannot be two or more equivalent electrons, for which in strong fields the values of all four quantum numbers coincide. If an electron exists in an atom for which all of these numbers have definite values, then this state is ‘occupied.’
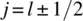
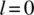
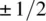
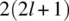
Table of contents
- Cover
- Title Page
- Table of Contents
- Preface
- 1 Historical Survey
- 2 Construction of Functions with a Definite Permutation Symmetry
- 3 Can the Pauli Exclusion Principle Be Proved?
- 4 Classification of the Pauli‐Allowed States in Atoms and Molecules
- 5 Parastatistics, Fractional Statistics, and Statistics of Quasiparticles of Different Kind
- Appendix A: Necessary Basic Concepts and Theorems of Group Theory
- Appendix B: The Permutation Group
- Appendix C: The Interconnection between Linear Groups and Permutation Groups
- Appendix D: Irreducible Tensor Operators
- Appendix E: Second Quantization
- Index
- End User License Agreement