
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About This Book
How can I use my HPLC/UHPLC equipment in an optimal way, where are the limitations of the technique? These questions are discussed in detail in the sequel of the successful "HPLC Expert" in twelve chapters written by experts in the respective fields. The topics encompass - complementary to the first volume - typical HPLC users' problems and questions such as gradient optimization and hyphenated techniques (LC-MS). An important key aspect of the book is UHPLC: For which analytical problem is it essential, what should be considered? Besides presentation of latest developments directly from the main manufacturers, also UHPLC users and independent service engineers impart their knowledge. Consistent with the target groups, the level is advanced, but the emphasis is on practical applications.
Frequently asked questions
Information
Chapter 1
When Should I Use My UHPLC as a UHPLC?
1.1 Introduction
Note
1.2 What Do I Want to Achieve and What Is a UHPLC Capable of?
1.2.1 What Do I Want to Achieve?
- Good separation: this can mean, firstly, sufficient resolution – separation between two critical peaks or possibly between 2 and 3 relevant peak pairs. Or, secondly, sufficient peak capacity – separation of many (or all?) – possibly chemically similar components, see Section 1.3.1
- Fast separation: short retention times; this often goes hand in hand with a low solvent consumption, see Section 1.3.2
- Sensitive measurement: decrease in the detection limit, which means an improvement in the relative mass sensitivity, see Section 1.3.3
- Robust conditions: reliable methods, which lead to the avoidance of repeat measurements and minimization of equipment downtime, see Section 1.3.4.
1.2.2 What Is a UHPLC Capable of?
Note
1.3 What Is Required from an HPLC Method?
1.3.1 Separate Well
- 1. I am really only interested in one or a few components. It is therefore a question of– according to my individual criteria – sufficient separation between the component of interest and an “interfering” component – in other words, ultimately on the separation of two peaks. The focus can be on the critical pair (e.g., main and secondary components), possibly on two to three more peak pairs. The criterion here is the resolution, and when simplified, it describes the distance between the peaks at the baseline. 1.1
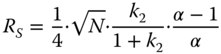
- where R = resolution, N = plate number (fundamentally defined for isocratic conditions), α = separation factor (formerly selectivity factor), and k = retention factor (formerly capacity factor k′).
- 2. I want to or have to separate “all” existing peaks sufficiently well, that is, when possible with baseline separation. In this case, the peak capacity comes into play. This is the total number of peaks that I can separate in a certain time with a sufficiently good resolution (commonly R = 1). The sum of all resolutions is often stated as a measure of the peak capacity. In the literature, one finds several formulas for the peak capacity, we consider here the two simplest: 1.2a
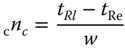
- or 1.2b
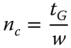
- where nc = peak capacity, tRl = retention time of the last peak, tRe = retention time of the first peak, w = peak width, and tG = gradient duration.
Note
- 1. Assume that two peaks elute with an α-value of 1.01. To achieve baseline separation of these two peaks, one would need about 160 000 plates. If the α-value could be increased from 1.01 to 1.10, for the same resolution, just less than 2000 plates would be required. Even a seemingly small improvement in the α-value from 1.01 to 1.05 means that instead of 160 000 plates, only about 6000 plates are necessary.
- 2. Further assume that we have a separation with the following values: k = 2, α = 1.05, and N = 9000. This results in a resolution of R = 0.76. This is not enough, and the resolution should be improved. To start with, the interactions can be increased, for example, through a more hydrophobic stationary phase or more water in the mobile phase. Assuming that the stronger interactions affect the two components equally, then the selectivity remains constant. The k-value increases from k = 2 to, for example, k = 6, and the resolution increases to R = 0.97. Alternatively, one could use a column with 15 000 plates, and the resolution improves to R = 0.98. Both measures are therefore correct; however, they are not particularly effective when it comes to significantly improving the resolution. If the α-value could be increased from 1.05 to 1.10, this would result in a resolution of R = 1.45. Let us finish the second example with the following observation: when two peaks ...
Table of contents
- Cover
- Title Page
- Copyright
- Table of Contents
- List of Contributors
- Foreword
- The Structure of “The HPLC-Expert 2”
- Chapter 1: When Should I Use My UHPLC as a UHPLC?
- Part I: Hardware and Software, Separation Modes, Materials
- Part II: Experience Reports, Trends
- About the Authors
- Index
- End User License Agreement