
Time-Resolved Mass Spectrometry
From Concept to Applications
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Time-Resolved Mass Spectrometry
From Concept to Applications
About this book
Time is an important factor in physical and natural sciences. It characterizes the progress of chemical and biochemical processes. Mass spectrometry provides the means to study molecular structures by detecting gas-phase ions with the unique mass-to-charge ratios. Time-resolved mass spectrometry (TRMS) allows one to differentiate between chemical states that can be observed sequentially at different time points. Real-time mass spectrometric monitoring enables recording data continuously with a specified temporal resolution. The TRMS approaches – introduced during the past few decades – have shown temporal resolutions ranging from hours down to microseconds and beyond.
This text covers the key aspects of TRMS. It introduces ion sources, mass analyzers, and interfaces utilized in time-resolved measurements; discusses the influence of data acquisition and treatment; finally, it reviews most prominent applications of TRMS – in the studies of reaction kinetics and mechanism, physicochemical phenomena, protein structure dynamics, biocatalysis, and metabolic profiling.
It will assist science and engineering students to gain a basic understanding of the TRMS concept, and to recognize its usefulness. In addition, it may benefit scientists who conduct molecular studies in the areas of chemistry, physics and biology.
Information
Chapter 1
Introduction
Time flies over us, but leaves its shadow behind.
1.1 Time in Chemistry
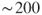
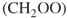
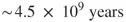
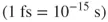
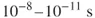
Table of contents
- Cover
- Title Page
- Copyright
- Table of Contents
- Author Biographies
- Preface
- Acknowledgments
- List of Acronyms
- Chapter 1: Introduction
- Chapter 2: Ion Sources for Time-resolved Mass Spectrometry
- Chapter 3: Mass Analyzers for Time-resolved Mass Spectrometry
- Chapter 4: Interfaces for Time-resolved Mass Spectrometry
- Chapter 5: Balancing Acquisition Speed and Analytical Performance of Mass Spectrometry
- Chapter 6: Hyphenated Mass Spectrometric Techniques
- Chapter 7: Microfluidics for Time-resolved Mass Spectrometry
- Chapter 8: Quantitative Measurements by Mass Spectrometry
- Chapter 9: Data Treatment in Time-resolved Mass Spectrometry
- Chapter 10: Applications in Fundamental Studies of Physical Chemistry
- Chapter 11: Application of Time-resolved Mass Spectrometry in the Monitoring of Chemical Reactions
- Chapter 12: Applications of Time-resolved Mass Spectrometry in the Studies of Protein Structure Dynamics
- Chapter 13: Applications of Time-resolved Mass Spectrometry in Biochemical Analysis
- Chapter 14: Final Remarks
- Index
- End User License Agreement
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app