
eBook - ePub
Cross Conjugation
Modern Dendralene, Radialene and Fulvene Chemistry
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Cross Conjugation
Modern Dendralene, Radialene and Fulvene Chemistry
Book details
Book preview
Table of contents
Citations
About This Book
Filling a gap in the chemical literature, this monograph provides a comprehensive overview of the fascinating and expanding field of cross-conjugated molecules, their chemistry, synthesis and properties.
The editors are world leading scientists in the field, and have assembled a team of experts to discuss different classes of cross-conjuagted molecules, as well as the use of cross-conjugation for organic synthesis and applications in electronic systems and material science.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Cross Conjugation by Henning Hopf,Michael S. Sherburn in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Synthesis of Dendralenes
Nicholas J. Green, Mehmet F. Saglam and Michael S. Sherburn
1.1 Introduction
The synthesis and study of conjugated polyenes has been at the heart of the chemical sciences ever since an appreciation of their structure began to develop. Of the five classes of conjugated alkenes that arise from the different possible modes of connectivity (Figure 1.1), some have received significantly more attention than others. The linear and cyclic classes featuring vicinal connections between alkene units – the linear polyenes 1 and annulenes 2 – are common structural motifs in naturally occurring compounds and contrived structures of industrial, commercial, and academic importance, and have hence been extensively synthesized and studied. Oligoalkenes with geminal connections between alkenes – cyclic radialenes 4 and acyclic dendralenes 5 – are yet to receive such attention, nor are “hybrid” structures featuring both geminal and vicinal connections, the fulvenes 3. There is, however, undoubtedly a growing interest in the subject of this chapter: the synthesis of dendralenes.
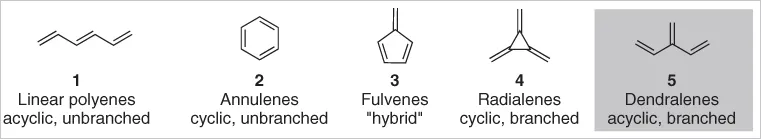
Figure 1.1 Fundamental conjugated hydrocarbons.
Dendralenes have been the subject of two comprehensive reviews [1, 2]. The first covers research in the area until 1984; and the second covers the period between 1984 and 2011. While it would be impossible to summarize the evolution of dendralene synthesis without some repetition of the key strategies found in each of these reviews, we seek to present the subject differently herein, by summarizing the best methods from both reviews, and placing emphasis on the significant work that has appeared between 2011 and the present. We also present the synthetic strategies in a new way, based on which carbon–carbon bonds of the dendralene are formed in the approach. Newly formed bonds are highlighted in bold, and should not be confused with wedged bonds, used to indicate stereochemistry. A broad measure of the synthetic power of a strategy is the number of bonds formed in the process, and we have therefore first highlighted strategies that form more than one bond per step [3]. This has allowed us insight into the strengths, weaknesses, and gaps present amongst current approaches. Our review covers examples in the literature up until April 2015, and we exclusively deal with the synthesis of the parent and substituted dendralenes, directing readers to other reviews or chapters of this book dealing with their closely related, cross-conjugated relatives (fulvenes [4], radialenes [5, 6], quinomethanes [7], etc.). We have not included related compounds that may be generated by substituting a carbon atom in the dendralene backbone with a similar unsaturated moiety, such as an alkyne or aromatic ring. We have also limited our survey to exclude cross-conjugated polymers, which have been reviewed elsewhere [8].
1.2 Multibond Forming Processes
1.2.1 Double Alkenylation Reactions
The double alkenylation approach (Scheme 1.1) has only been exploited relatively recently, most probably because of the rise to prominence of cross-coupling methodologies in recent times. The first double cross-couplings between 1,1-dihaloalkenes and metalloalkenes were isolated examples appearing in 1998 [9] and 2000 [10]. In 2002, Oh and Lim [11] reported a series of double Suzuki–Miyaura reactions between a 1,1-dibromoalkene 6 and alkenyl boronic acids 7 (Scheme 1.2). In 2007 and 2008, the Sherburn research group reported syntheses of substituted [3]dendralenes [12] and the state-of-the-art synthesis of [5]dendralene [13] respectively, transforming a 1,1-dihaloalkene via double Negishi or Kumada cross-couplings to incorporate one alkenyl substituent (9 or 12) twice, and also, in the former case, the related stepwise, stereoselective Stille couplings to form unsymmetrically substituted, chiral [3]dendralenes 16 (Scheme 1.2). An application of this stepwise approach en route to the natural product triptolide [14] highlighted that when using two different metalloalkene cross-coupling partners, complete control of the stereochemistry of the resulting alkene is sometimes unattainable. Thus, most successful applications of this method incorporate two identical alkenes, so no issues of stereochemistry arise. A recent example is the synthesis ...
Table of contents
- Cover
- Title Page
- Copyright
- Table of Contents
- List of Contributors
- Preface
- Chapter 1: Synthesis of Dendralenes
- Chapter 2: The Diene-Transmissive Hetero-Diels–Alder Reaction
- Chapter 3: The Nazarov Cyclization of Cross-Conjugated Ketones
- Chapter 4: [n]Radialenes
- Chapter 5: Oxocarbons, Pseudo-oxocarbons, and Squaraines
- Chapter 6: Recent Developments in Fulvene and Heterofulvene Chemistry
- Chapter 7: Constructing Molecular Complexity and Diversity by Cycloaddition Reactions of Fulvenes
- Chapter 8: Cross-Conjugation and Electronic Structure in TTF Analogs
- Chapter 9: Cross-Conjugation in Expanded Systems
- Chapter 10: Transition Metal Complexes of Cross-Conjugated π Systems
- Chapter 11: Cross-Conjugation and Quantum Interference
- Chapter 12: Cross-Conjugation in Synthesis
- Author Index
- Subject Index
- End User License Agreement