
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Chemical Equilibria
About this book
The book offers advanced students, in 7 volumes, successively characterization tools phases, the study of all types of phase, liquid, gas and solid, pure or multi-component, process engineering, chemical and electrochemical equilibria, the properties of surfaces and phases of small sizes. Macroscopic and microscopic models are in turn covered with a constant correlation between the two scales. Particular attention was given to the rigor of mathematical developments.
Besides some very specialized books, the vast majority of existing works are intended for beginners and therefore limited in scope. There is no obvious connection between the two categories of books, general books does not go far enough in generalizing concepts to enable easy reading of advanced literature. The proposed project aims to give readers the ability to read highly specialized publications based on a more general presentation of the different fields of chemical thermodynamics. Consistency is ensured between the basic concepts and applications. So we find, in the same work, the tools, their use and comparison, for a more general macroscopic description and a microscopic description of a phase.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Physico-Chemical Transformations and Equilibria
1.1. Characteristic parameters of physico-chemical transformations
- – physical transformations where there is no alteration of the chemical nature of the species but there is a modification of their phases;
- – chemical transformations or reactions, where there is a modification of the chemical nature of the species present, and where that modification may or may not be accompanied by a phase change.
1.1.1. Balance equation of a transformation
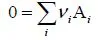
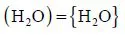
| Symbol | Meaning | Symbol | Meaning |
| {A} | Pure A in a gaseous phase | {{A}} | A in a gaseous mixt... |
Table of contents
- Cover
- Table of Contents
- Title
- Copyright
- Preface
- Notations and Symbols
- 1: Physico-Chemical Transformations and Equilibria
- 2: Properties of States of Physico-Chemical Equilibrium
- 3: Molecular Chemical Equilibria
- 4: Determination of the Values Associated with Reactions - Equilibrium Calculations
- APPENDICES
- Bibliography
- Index
- End User License Agreement
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app