
eBook - ePub
Multicomponent Reactions in Organic Synthesis
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Multicomponent Reactions in Organic Synthesis
About this book
Comprehensive and up-to-date, this book focuses on the latest advances in the field, such as newly developed techniques, more environmentally benign processes, broadened scopes, and completely novel MCRs. In addition to carbene-promoted MCRs and frequently applied metal-catalyzed MCRs, it also covers recently developed catalytic enantioselective variants as well as MCR in drug discovery and for the synthesis of heterocyclic molecules and macrocycles.
Edited by the leading experts and with a list of authors reading like a "who's who" in multicomponent reaction chemistry, this is definitely a must-have for every synthetic organic chemist as well as medicinal chemists working in academia and pharmaceutical companies.
Edited by the leading experts and with a list of authors reading like a "who's who" in multicomponent reaction chemistry, this is definitely a must-have for every synthetic organic chemist as well as medicinal chemists working in academia and pharmaceutical companies.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
General Introduction to MCRs: Past, Present, and Future
Alexander Dömling and AlAnod D. AlQahtani
1.1 Introduction
Multicomponent reactions (MCRs) are generally defined as reactions in which three or more starting materials react to form a product, where basically all or most of the atoms contribute to the newly formed product [1]. Their usefulness can be rationalized by multiple advantages of MCRs over traditional multistep sequential assembly of target compounds. In MCRs, a molecule is assembled in one convergent chemical step in one pot by simply mixing the corresponding starting materials as opposed to traditional ways of synthesizing a target molecule over multiple sequential steps. At the same time, considerably complex molecules can be assembled by MCRs. This has considerable advantages as it saves precious time and drastically reduces effort.
MCRs are mostly experimentally simple to perform, often without the need of dry conditions and inert atmosphere. Molecules are assembled in a convergent way and not in a linear approach using MCRs. Therefore, structure–activity relationships (SARs) can be rapidly generated using MCRs, since all property-determining moieties are introduced in one step instead of sequentially [2]. Last but not least, MCRs provide a huge chemical diversity and currently more than 300 different scaffolds have been described in the chemical literature. For example, more than 40 different ways to access differentially substituted piperazine scaffolds using MCRs have been recently reviewed [3].
Although MCR chemistry is almost as old as organic chemistry and was first described as early as 1851, it should be noted that early chemists did not recognize the enormous engineering potential of MCRs. However, it took another >100 years until Ivar Ugi in a strike of a genius discovered his four-component condensation and also recognized the enormous potential of MCRs in applied chemistry (Figure 1.1) [4].
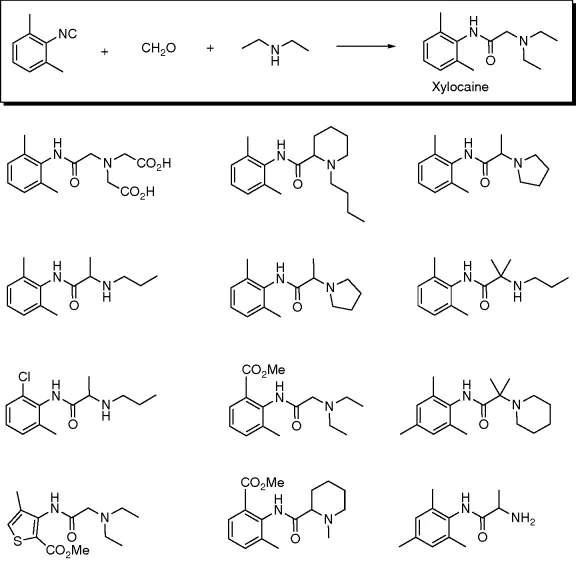
Figure 1.1 A three-component reaction toward the local anesthetic xylocaine and the first combinatorial library of small molecules proposed by Ivar Ugi in the 1960.
1.2 Advances in Chemistry
Many MCRs have been described in the past one and a half century and recently not many fundamental advances in finding new MCRs have been made [5–7]. A strategy to enhance the size and diversity of current MCR chemical space is the concept of combining a MCR and a subsequent secondary reaction, also known as postcondensation or Ugi–deprotection–cyclization (UDC) [2]. Herein, bifunctional orthogonally protected starting materials are used and ring cyclizations can take place in a secondary step upon deprotection of the secondary functional groups. Many different scaffolds have been recently described using this strategy. One example is shown in Figure 1.2. It is based on a recently discovered variation of the Ugi reaction of α-amino acids, oxo components, and isocyanides, now including primary and secondary amines [8–10].

Figure 1.2 Discovery of the Ugi-5C-4CR variation employing unprotected α-amino acids, oxo components, primary or secondary amines, and isocyanides, and the synthesis of several heterocyclic scaffolds using orthogonally protected bifunctional starting materials. Generalized scaffolds are shown in color, and synthesized examples in black and white.
1.3 Total Syntheses
While the Bucherer–Bergs and the related Strecker synthesis are well-established methods for the one-pot synthesis of natural and unnatural amino acids, the complex antibiotic penicillin was synthesized 50 years ago in a highly convergent approach by Ivar Ugi by using two MCRs, the Asinger reaction and his own reaction (Figure 1.3) [11]. Other recent natural product targets using MCR as a key step in their synthesis are also shown in Figure 1.3. Although early example of the advantageous use of MCR in the conscious total synthesis of complex natural products leads the way, its use has been neglected for decades and only recently realized by a few organic chemists [12–17].

Figure 1.3 (a) The union of the Asinger-4CR and the Ugi-4CR allows for the convergent and fast assembly of 6-aminopenicillanic acid natural product. (b) Recent synthetic targets of MCR natural product chemistry.
1.4 Applications in Pharmaceutical and Agrochemical Industry
Two decades ago, MCR chemistry was almost generally neglected in pharmaceutical and agro industry. The knowledge of these reactions was often low and it was generally believed that MCR scaffolds are associated with useless drug-like properties (absorption, distribution, metabolism, excretion, and toxicity (ADMET)). Now MCR technology is widely recognized for its impact on drug discovery projects and is strongly endorsed by industry as well as academia [18]. An increasing number of clinical and marketed drugs were discovered and assembled by MCR since then (Figure 1.4). Examples include nifedipine (Hantzsch-3CR), praziquantel, or Zetia™. Two oxytocin receptor antagonists for the treatment of preterm birth and premature ejaculation, epelsiban and atosiban, are currently undergoing human clinical trials. They are both assembled by the classical Ugi MCR [19–21]. Interestingly, they show superior activity for the oxytocin receptor and selectivity toward the related vasopressin receptors than the peptide-based compounds currently used clinically. Perhaps against the intuition of many medicinal chemists, the Ugi diketopiperazines are orally bioavailable, while the currently used peptide derivatives are i....
Table of contents
- Cover
- Related Titles
- Title Page
- Copyright
- Preface
- List of Contributors
- Chapter 1: General Introduction to MCRs: Past, Present, and Future
- Chapter 2: Discovery of MCRs
- Chapter 3: Aryne-Based Multicomponent Reactions
- Chapter 4: Ugi–Smiles and Passerini–Smiles Couplings
- Chapter 5: 1,3-Dicarbonyls in Multicomponent Reactions
- Chapter 6: Functionalization of Heterocycles by MCRs
- Chapter 7: Diazoacetate and Related Metal-Stabilized Carbene Species in MCRs
- Chapter 8: Metal-Catalyzed Multicomponent Synthesis of Heterocycles
- Chapter 9: Macrocycles from Multicomponent Reactions
- Chapter 10: Multicomponent Reactions under Oxidative Conditions
- Chapter 11: Allenes in Multicomponent Synthesis of Heterocycles
- Chapter 12: Alkynes in Multicomponent Synthesis of Heterocycles
- Chapter 13: Anhydride-Based Multicomponent Reactions
- Chapter 14: Free-Radical Multicomponent Processes
- Chapter 15: Chiral Phosphoric Acid-Catalyzed Asymmetric Multicomponent Reactions
- Index
- End User License Agreement
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Multicomponent Reactions in Organic Synthesis by Jieping Zhu, Qian Wang, Meixiang Wang, Jieping Zhu,Qian Wang,Meixiang Wang in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.