
- English
- ePUB (mobile friendly)
- Available on iOS & Android
The Organometallic Chemistry of N-heterocyclic Carbenes
About This Book
The Organometallic Chemistry of N-heterocyclic Carbenes describes various aspects of N-heterocyclic Carbenes (NHCs) and their transition metal complexes at an entry level suitable for advanced undergraduate students and above.
The book starts with a historical overview on the quest for carbenes and their complexes. Subsequently, unique properties, reactivities and nomenclature of the four classical NHCs derived from imidazoline, imidazole, benzimidazole and 1, 2, 4-triazole are elaborated. General and historically relevant synthetic aspects for NHCs, their precursors and complexes are then explained. The book continues with coverage on the preparation and characteristics of selected NHC complexes containing the most common metals in this area, i.e. Ni, Pd, Pt, Ag, Cu, Au, Ru, Rh and Ir. The book concludes with an overview and outlook on the development of various non-classical NHCs beyond the four classical types.
Topics covered include:
- Stabilization, dimerization and decomposition of NHCs
- Stereoelectronic properties of NHCs and their evaluation
- Diversity of NHCs
- Isomers of NHC complexes and their identification
- NMR spectroscopic signatures of NHC complexes
- normal, abnormal and mesoionic NHCs
The Organometallic Chemistry of N-heterocyclic Carbenes is an essential resource for all students and researchers interested in this increasingly important and popular field of research.
Frequently asked questions
Information
1
General Introduction
1.1 Definition of Carbenes
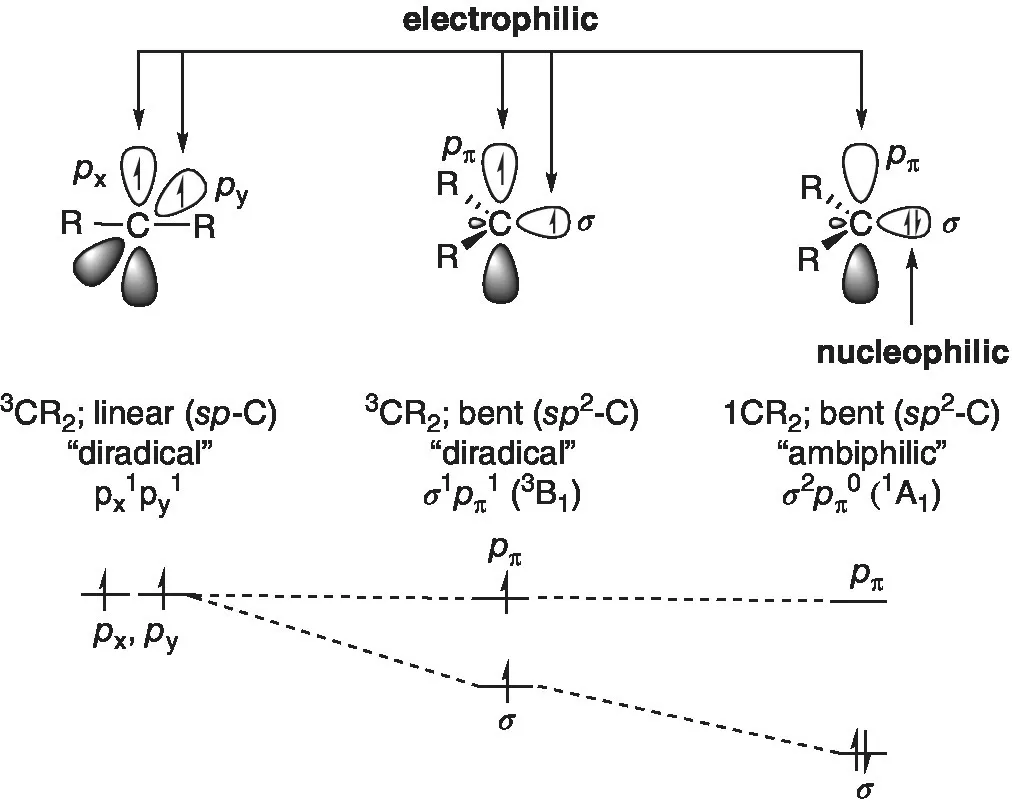
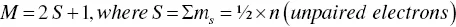
Definition of spin multiplicity for the determination of singlet and triplet state.
Table of contents
- Cover
- Title Page
- Table of Contents
- Foreword
- Preface
- List of Abbreviations and Definitions
- 1 General Introduction
- 2 General Properties of Classical NHCs and Their Complexes
- 3 Synthetic Aspects
- 4 Group 10 Metal(0)‐NHC Complexes
- 5 Group 10 Metal(II)‐NHC Complexes
- 6 Group 11 Metal‐NHC Complexes
- 7 Ruthenium, Rhodium, and Iridium Metal‐NHC Complexes
- 8 Beyond Classical N‐heterocyclic Carbenes I
- 9 Beyond Classical N‐heterocyclic Carbenes II
- Index
- End User License Agreement