
Oral Bioavailability Assessment
Basics and Strategies for Drug Discovery and Development
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Oral Bioavailability Assessment
Basics and Strategies for Drug Discovery and Development
About This Book
Specifically geared to personnel in the pharmaceutical and biotechnology industries, this book describes the basics and challenges of oral bioavailability – one of the most significant hurdles in drug discovery and development. • Describes approaches to assess pharmacokinetics and how drug efflux and uptake transporters impact oral bioavailability
• Helps readers reduce the failure rate of drug candidates when transitioning from the bench to the clinic during development
• Explains how preclinical animal models – used in preclinical testing – and in vitro tools translate to humans, which is an underappreciated and complicated area of drug development
• Includes chapters about pharmacokinetic modelling, the Biopharmaceutics Drug Disposition Classification System (BDDCS), and the Extended Clearance Classification System (ECCS)
• Has tutorials for applying strategies to medicinal chemistry practices of drug discovery/development
Frequently asked questions
Information
CHAPTER 1
DRUG PHARMACOKINETICS AND TOXICOKINETICS
1.1 INTRODUCTION
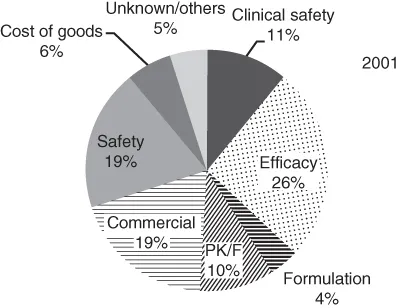
- select compounds with the maximum potential of reaching the target;
- determine the appropriate route of administration to deliver the drug (typically oral);
- understand how the drug blood levels relate to efficacy or toxicity in order to choose efficacious and safe doses;
- facilitate appropriate dose sections for rodent and/or nonrodent species in toxicology testing and drug safety evaluation;
- decide on the frequency and duration of dosing in order to maintain adequate drug concentration at target for disease modification; and
- accurately predict the PK in humans profile prior to clinical studies.
Table of contents
- COVER
- WILEY SERIES ON PHARMACEUTICAL SCIENCE AND BIOTECHNOLOGY: PRACTICES, APPLICATIONS, AND METHODS
- TITLE PAGE
- COPYRIGHT
- TABLE OF CONTENTS
- DEDICATION
- CHAPTER 1: DRUG PHARMACOKINETICS AND TOXICOKINETICS
- CHAPTER 2: GIT ANATOMY AND PHYSIOLOGY AND DRUG ORAL BIOAVAILABILITY: IMPACT OF SPECIES DIFFERENCES
- CHAPTER 3: DRUG ROUTES OF EXCRETION
- CHAPTER 4: PHYSICOCHEMICAL AND BIOPHARMACEUTICAL PROPERTIES THAT AFFECT DRUG ABSORPTION OF COMPOUNDS ABSORBED BY PASSIVE DIFFUSION
- CHAPTER 5: PHYSICOCHEMICAL AND BIOPHARMACEUTICAL FACTORS AFFECTING HEPATIC/INTESTINAL FIRST-PASS EFFECT
- CHAPTER 6: IMPACT OF INTESTINAL EFFLUX TRANSPORTERS ON ORAL ABSORPTION
- CHAPTER 7: IMPACT OF INFLUX TRANSPORTERS ON DRUG ABSORPTION
- CHAPTER 8: EXTENDED CLEARANCE CLASSIFICATION SYSTEM (ECCS) AND ITS UTILITY IN PREDICTING CLEARANCE RATE-DETERMINING STEP IN DRUG DISCOVERY
- CHAPTER 9: IN VITRO AND IN SITU APPROACHES TO MEASURE INTESTINAL PERMEABILITY AND EFFLUX TRANSPORTERS
- CHAPTER 10: IN SILICO APPROACHES TO PREDICT INTESTINAL PERMEABILITY
- CHAPTER 11: IN VIVO PRECLINICAL APPROACHES TO DECONVOLUTE THE CONTRIBUTION OF FIRST-PASS EFFECT FROM ORAL ABSORPTION
- CHAPTER 12: IN VITRO APPROACHES TO ASSESS HEPATIC METABOLISM AND FIRST-PASS EFFECT
- CHAPTER 13: THE UTILITY OF ECCS AS A ROADMAP TO IMPROVE ORAL BIOAVAILABILITY OF NEW MOLECULAR ENTITIES: INDUSTRIAL PERSPECTIVE
- INDEX
- END USER LICENSE AGREEMENT