
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About This Book
Compiling all the information available on the topic, this ready reference covers all important aspects of iron oxides.
Following a preliminary overview chapter discussing iron oxide minerals along with their unique structures and properties, the text goes on to deal with the formation and transformation of iron oxides, covering geological, synthetic, and biological formation, as well as various physicochemical aspects. Subsequent chapters are devoted to characterization techniques, with a special focus on X-ray-based methods, magnetic measurements, and electron microscopy alongside such traditional methods as IR/Raman and Mossbauer spectroscopy. The final section mainly concerns exciting new applications of magnetic iron oxides, for example in medicine as microswimmers or as water filtration systems, while more conventional uses as pigments or in biology for magnetoreception illustrate the full potential.
A must-read for anyone working in the field.
Frequently asked questions
Information
Chapter 1
Introduction
1.1 Iron Oxides: From Nature to Applications
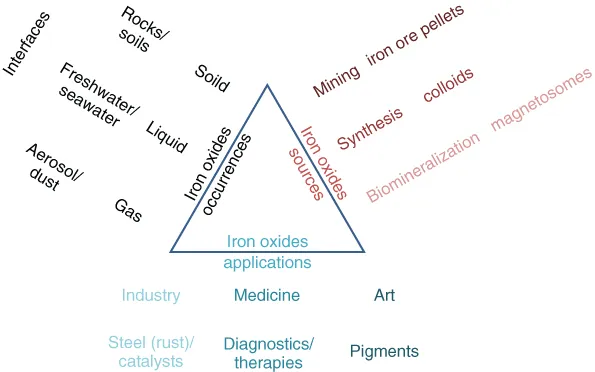

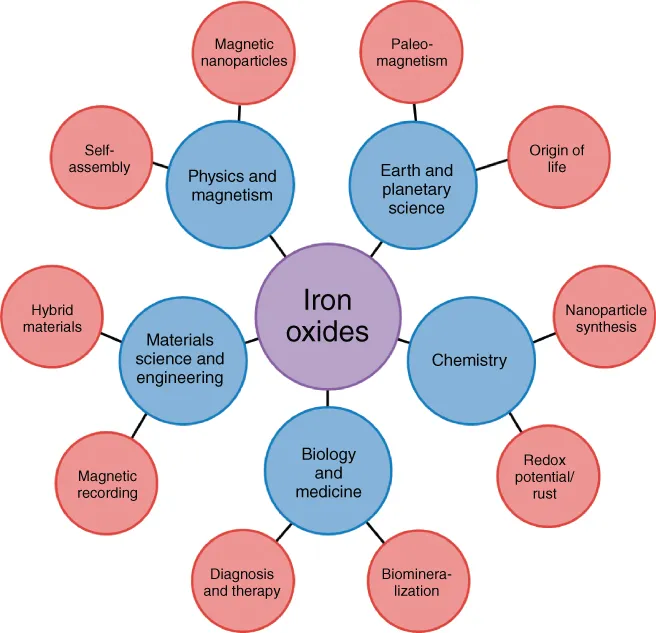
1.2 A Very Brief Overview of the Iron Oxides and How They Found Names
| Iron oxides | Iron oxyhydroxides | Iron hydroxides | |
| Fe(II) compounds | Wüstite FeO [4, 5] | “White rust” – Fe(OH)2 [6, 7] | |
| Fe(II)-Fe(III) compounds | Magnetite Fe3O4 [8] | “Green rusts” – Fougèrite [Fe2+4Fe3+2(OH)12][CO3]·3H2O [9] | |
| Fe(III) compounds | Hematite α-Fe2O3 [8] | Goethite α-FeOOH [8] | Bernalite Fe(OH)3 [10] |
| β-Fe2O3 [11] | Akaganéite β-FeOOH [12] | ||
| Maghemite γ-Fe2O3 [13] | Lepidocrocite γ-FeOOH [14] | ||
| δ-Fe2O3 [15] | Feroxyhyte δ-FeOOH [16–18] | ||
| ϵ-Fe2O3 [19] | |||
| Ferrihydrite 5Fe2O3·9H2O [20, 21] | |||
| Schwertmannite Fe8O8(OH)6(SO4)·nH2O [8, 22] |
Table of contents
- Cover
- Related Titles
- Title Page
- Copyright
- Table of Contents
- List of Contributors
- Foreword
- Preface
- Chapter 1: Introduction
- Part I: Formation, Transformation
- Part II: Characterization Techniques
- Part III: Applications
- Index
- End User License Agreement