
Basic Sciences for Dental Students
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Basic Sciences for Dental Students
About this book
The 'all-in-one' solution to mastering basic sciences in preclinical dentistry
Basic Sciences for Dental Students is a cutting edge textbook specifically designed to support the needs of early years undergraduate dental students. Written by leaders in dental education and active oral and dental researchers involved with student assessment, the text explains the basic science that underpins the dental curriculum in undergraduate dental courses worldwide.
Specifically related to dentistry and future clinical practice, chapters cover all of the introductory subjects that students need to know – biomolecules, cell biology, tissues of the body, cardiovascular, circulatory and pulmonary systems, the nervous system, immunology, oral microbiology, pathology, head and neck anatomy, tooth development, craniofacial development, saliva, and dental materials.
Key features:
- Provides the basic science that underpins the early years of a dental curriculum
- Specifically tailored towards dentistry and future clinical practice
- Written by leaders in dental education and active oral and dental researchers
- Includes learning objectives and clinical relevance boxes throughout
- Self-assessment questions and downloadable figures are hosted on a companion website
Basic Sciences for Dental Students is an indispensable resource for undergraduate dental students, especially those in the early years of their studies. It is also a useful revision tool for postgraduate MJDF and MFDS examinations and overseas candidates sitting their OREs.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Biomolecules
Learning Objectives
- To understand the basis of molecular structure and bonding.
- To outline the basic structure and function of proteins, carbohydrates, lipids and nucleic acids.
- To be able to describe the biological role of enzymes and explain how their activity is regulated.
- To understand basic energy‐yielding pathways and how they are controlled.
Clinical Relevance
Introduction
Biological Bonding
Water, Water Everywhere
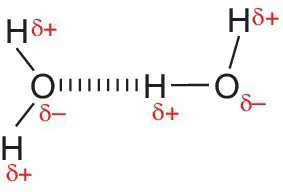
Amino Acids and Proteins
Table of contents
- Cover
- Title Page
- Table of Contents
- List of Contributors
- About the Companion Website
- 1 Biomolecules
- 2 Cell Biology
- 3 Tissues of the Body
- 4 The Cardiovascular, Circulatory and Pulmonary Systems
- 5 The Nervous System
- 6 Introduction to Immunology
- 7 Oral Microbiology
- 8 Introduction to Pathology
- 9 Head and Neck Anatomy
- 10 Tooth Development, Tooth Morphology and Tooth‐Supporting Structures
- 11 Craniofacial Development
- 12 Saliva and Salivary Glands
- 13 Introduction to Dental Materials
- Index
- End User License Agreement
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app