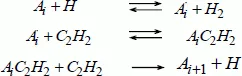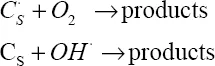![]()
1
Generation of Multiphase Flows
Multiphase mixtures exist in the natural state, and are highly varied. If we wish to generate multiphase flows for research or for industrial purposes, it is not always easy to do, and often necessitates a profound technological study. In this chapter, we examine the issue of the generation of particular two-phase mixtures: dusty gases, fogs and bubbling flows. The production of mists for combustion is a complex problem, with numerous steps, usually divided into primary atomization and secondary atomization.
1.1. Creation of suspensions of solid particles in a gaseous phase
Here we present two very different examples of the generation of flows with solid particles.
The first case study is designed as a micro-gravity examination of the combustion of a suspension of wheat starch particles.
The second study concerns the formation of soot.
1.1.1. Creation of a homogeneous suspension of starch particles
Let us look at the example of the creation, for research purposes1, of a suspension of wheat starch particles of 20µm average diameter in a vessel [VEY 03, BOZ 04, TAK 09, BOZ 10].
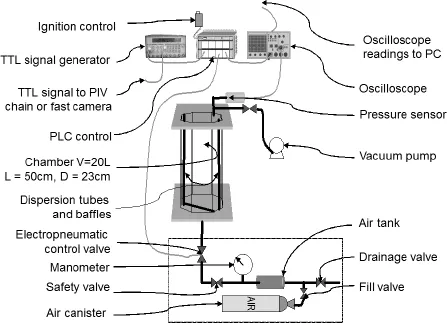
Consider a specific setup of apparatus, comprising a transparent cylindrical chamber with an octagonal cross-section and short relative length (volume = 20 liters; length/transverse dimension ≡ 2.2). The particles are dispersed by means of a turbulent flow created by the discharging of an auxiliary tank (see Figure 1.1). The experiments are conducted with starch/air mixtures at concentrations lower than 400g/m3.
Figure 1.1. Experimental setup for the generation of a suspension of solid particles [VEY 03] (diagram reproduced with kind permission from the authors)
The recordings, made by fast imaging techniques (LDV and PIV2), of the evolution over time of the aerodynamic state of the mixture and the distribution of particles in the chamber indicate that the time needed to obtain an optimal mixture (minimal velocity and turbulence intensity, quasi-homogeneous concentration) is between 500 and 700 ms.
1.1.2. Soot formation
Soot particles are formed during the combustion of carbonate species. Their nucleation and growth, and their interaction with the flow of reagents, have been studied by many authors. In particular, we can cite the case of the Emmons problem, involving a boundary layer with a flame above a flat plate [EMM 56, WIL 85] (see section 5.3.2). The relatively recent effort to take account of soot particles in this problem has required appropriate mechanisms to be developed [BOC 94, FRE 89, FRE 94, WAN 96, ZHA 05, SGR 07], and it has been necessary to take into account the soot’s interaction with the flow and the ablative surface [WAN 07, LEG 05b, LEG 06, LEG 09].
Dorey [DOR 12] offers a complete review of this complex problem. There are four distinct processes involved:
1) nucleation;
2) surface growth;
3) oxidation;
4) coagulation.
Let us now summarize these steps as simply as possible.
Nucleation occurs because of the degradation of gaseous hydrocarbons by pyrolysis, which leads to the formation of smaller carbonate species, such as acetylene C2H2 and benzene. A wide variety of large, flat molecules known as PAHs (Polycyclic Aromatic Hydrocarbons) can be created by the coming together of these species.
The formulation proposed by Frenklach and Wang [FRE 94] for the nucleation of PAHs is given by the system of chemical reactions [1.1]:
In this system, the species Ai represents a molecule containing i aromatic rings fused together. Ai⋅ is the associated radical and AiC2H2⋅ is the radical obtained after the addition of acetylene.
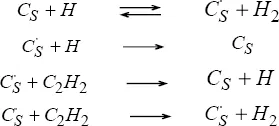
For the
growth of surface area of soot, Frenklach and Wang [FRE 94] present the analogy with the HACA mechanism (addition of acetylene by the reaction: “H-Abstraction/C
2H
2-Addition”) for the surface of the PAHs, and obtain the system
[1.2], where
CS represents a soot particle and
the radical associated therewith:
Oxidation is characterized by the two reactions:
The species O, CO2 and H2O may also be involved, but play a lesser role in the oxidation process [XUE 03].
Coagulation results from collisional interactions between the small particles thus formed. The process involves coalescence (fusion of two particles into one) and agglomeration (particles which stick to one another).
1.2. Creation of suspensions of bubbles in a liquid
Bubbly flows have been studied for a long time, hence we now have a deep understanding of their behavior, particularly in pipes [CLI 78, NIG 91] and around submarine propellers.
The nucleation of bubbles of vapor can hinder heat transfer when pipes are cooled. This is a major problem for the cooling circuits of rocket engines. For example, the walls of the nozzles of cryogenic rocket engines are lined with tubes filled with liquid hydrogen, and the appearance of bubbles...