
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Peptide Chemistry and Drug Design
Book details
Book preview
Table of contents
Citations
About This Book
This book focuses on peptides as drugs, a growing area of pharmaceutical research and development. It helps readers solve problems of discovering, developing, producing, and delivering peptide-based drugs.
•Identifies promising new areas in peptide drug discovery
•Includes chapters on discovery from natural sources, metabolic modification, and drug delivery
•Overviews separation methods and techniques for analysis, bond formation, and purification
•Offers readers both a professional reference and a text or resource for graduate-level students
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Peptide Chemistry and Drug Design by Ben M. Dunn in PDF and/or ePUB format, as well as other popular books in Medicine & Pharmacology. We have over one million books available in our catalogue for you to explore.
Information
1
Peptide Therapeutics
Nader Fotouhi
Global Alliance for TB Drug Development, Research and Development, New York, NY, USA
1.1 History of Peptides as Drugs
The advent of molecular biology and our understanding of the physiological and pathological functions of peptides, coupled with advances in synthetic methodologies and peptidomimetics, marked the beginning of a new era in peptide and protein therapeutics, with the vision that there should be no limit to what can be produced as therapeutics. During that period a number of great peptide drugs such as Sandostatin, Lupron, Copaxone, and Zoladex were developed with great therapeutic benefit. The number of approved peptide drugs, however, remains low.
It was not until the last decade that we have seen a significant surge in the number of peptide therapeutics on the market (Figure 1.1). While 10 peptides were approved between 2001 and 2010, the current decade has thus far witnessed the approval of six new peptide therapeutics – a remarkable yearly increase [1, 2]. The number of peptides in development is also steadily growing roughly doubling every decade (Figures 1.2 and 1.3), and there are 400–600 peptides in preclinical studies. This is due to the advances made in our understanding of peptide stability, peptide synthesis, and formulation over the last three decades. Although the market share of peptide drugs is still relatively small (about 2% of the global market for all drugs), the approval rate for peptide drugs is twice as fast as the rate for small molecules, and the market is growing similarly at a rate that is twice the global drug market [3, 4]. While encouraging, the potential for peptide therapeutics is far greater than what it is today.
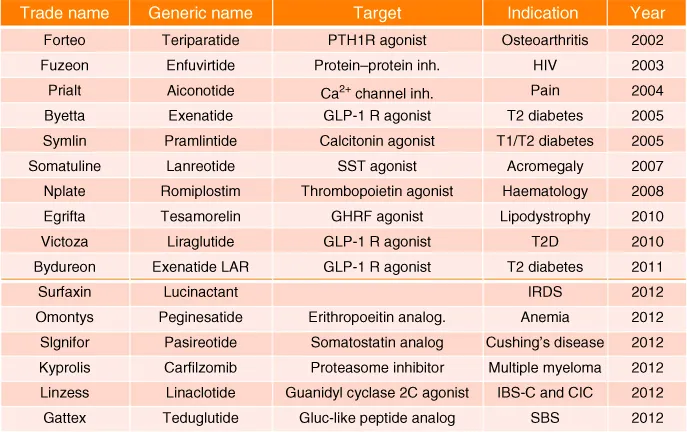
Figure 1.1 Peptide therapeutics marketed since 2002.
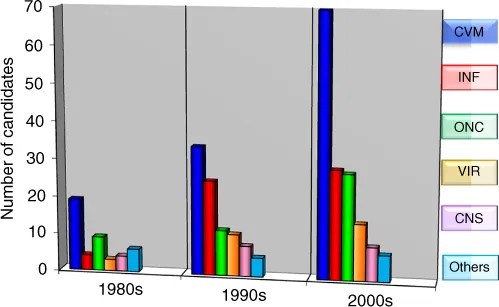
Figure 1.2 Peptides in development over the last three decades.
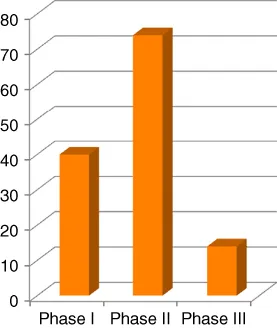
Figure 1.3 Peptides in clinical trials in 2013.
1.2 Factors Limiting The Use of Peptides in The Clinic
A number of factors have thus far limited the explosion that needs to happen in the peptide field. With the exception of a few peptides, the approved drugs so far target the extracellular compartment, and thus have to compete with biologics. Of the extracellular targets, GPCRs represent the major class, and in most cases, the peptides are agonist. GLP-1 represents one-third of these GPCR targets. We have seen a great advance in extending the circulating half-life of the peptides through the use of unnatural amino acids and formulation technologies, but have not yet reached the half-life achieved by antibodies. The delivery of peptides is still in the great majority of cases limited to i.v. (intravenous), s.c. (subcutaneous), or intranasal. Finally, safety is still a concern as better tissue selectivity is required.
To dramatically heighten their impact, peptides need to access the intracellular space to target protein–protein interactions. These interactions represent a vast source of potential targets with significant biological impact (there are estimated 300,000 such interactions in the cell), and will not in the majority of cases be modulated by small molecules. Peptides and biologics, given their relative size and ability to bind to extended surface areas, are the perfect candidates to inhibit protein–protein interactions. The duration of action of peptides needs to be extended, and while peptides are inherently selective against their targets, they need to more selectively distribute to the desired tissue. Finally, the route of administration needs to be expanded to include oral delivery.
1.3 Advances that have Stimulated The Use of Peptides as Drugs
The many great technological advances that started over a decade ago in drug delivery, peptide design, and synthesis are now maturing, and will undoubtedly address these key challenges and revolutionize the field over the next decades. Many of the technological advances are already proving that it is possible to make peptides permeable to cells, target tissues, have longer half-lives, and be orally bioavailable.
The discovery that certain peptides can penetrate cells and can, therefore, be an effective therapeutic on their own or alternatively bring other drugs into cells allowed for the first time to imagine targeting the intracellular compartment (Figures 1.4 and 1.5) [5]. HIV-enveloped protein tat was one of the first to be recognized for its cell-penetrating ability and, therefore, its potential use to carry bioactive cargo into the cell [6]. Since 2004, more than 200 peptides carried into cells by tat or other naturally occurring cell-penetrating peptides (CPPs) have been in various phases of development [7]. However, the more recent advances in the understanding of how these peptides cross the cell membrane through endocytosis and/or macropinocytosis [8] has allowed the generation of CPPs with intrinsic biological activity [9–12]. It is now possible to take a CPP sequence and synthetically modify it to introduce the key amino acids of an effector peptide into its sequence and create potent peptide antagonists of an intracellular protein–protein interaction with good pharmacokinetic properties [13].
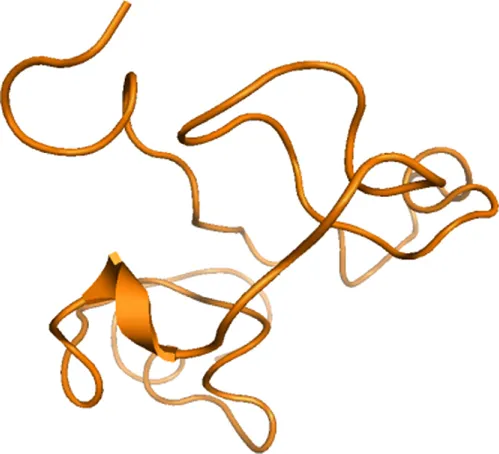
Figure 1.4 HIV Tat.
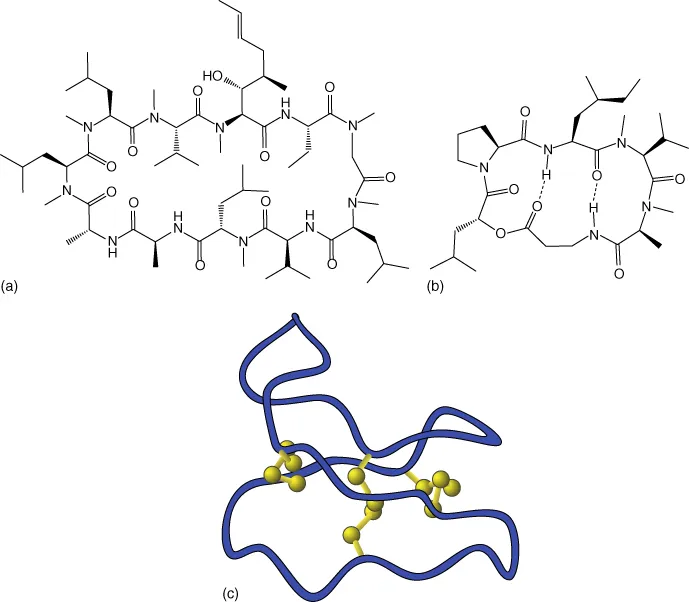
Figure 1.5 Orally stable and bioavailable peptides (a) Cyclosporin. (b) Destruxin. (c) Kalata B.
1.4 Development of Peptide Libraries
By looking at the list of CPPs in development, one realizes that they are single cases and have to be synthetically prepared and modified to impart some of the desired stability to be a useful therapeutic. It is hard to compete with the screening of the millions of small molecule compounds in various pharmaceutical companies and more recently in many academic centers.
Until now, the available technologies to screen large libraries of peptides of significant length (possessing secondary structure) would only allow us to generate large libraries of natural amino acid sequences through phage display, and if unnatural amino acids were to be introduced, it had to be done with conventional synthetic methodology, and thus be limited to very low numbers of peptides that can be prepared and screened.
Indeed, over the last decade, there has been an explosion of very elegant technologies that now allow the generation of large to extremely large libraries of linear and macrocyclic peptides with unnatural amino acids and unnatural linkers. For the first time, it is possible to engineer stability, cell permeability, and possibly oral bioavailability at once and screen for the desired properties very rapidly. These major advancements have resulted in the generation of a number of companies that are pushing the limits of these technologies to rapidly screen and identify novel peptide therapeutics against protein–protein interaction targets (Figure 1.5).
Ensemble therapeutics utilizing their DNA-programmed chemistry can generate million-member libraries of small macrocycles with MW of 500–1500. On screening these libraries, they have identified potent and orally bioavailable small molecule inhibitors of IL17 [14]. Through medicinal chemistry optimization, they have now identified picomolar inhibitors with good properties [15]. PeptiDream utilizing Professor Suga's mRNA display technology [16] are generating up to trillion-member libraries of larger macrocycles mimicking cyclosporin. These peptides contain a combination of natural, unnatural, and N-methyl amino acids and exhibit good physicochemical properties and membrane permeability [17]. Ra Pharmaceuticals also uses a mRNA display technology developed by Jack Shoztac to generate very large libraries of macrocycles containin...
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- List of Contributors
- Chapter 1: Peptide Therapeutics
- Chapter 2: Methods for The Peptide Synthesis and Analysis
- Chapter 3: Peptide Design Strategies for G-Protein Coupled Receptors (GPCR)
- Chapter 4: Peptide-Based Inhibitors of Enzymes
- Chapter 5: Discovery of Peptide Drugs as Enzyme Inhibitors and Activators
- Chapter 6: Discovery of Peptide Drugs From Natural Sources
- Chapter 7: Modification of Peptides to Limit Metabolism
- Chapter 8: Delivery of Peptide Drugs
- Index
- End User License Agreement