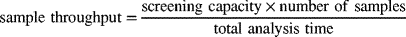1.1 Introduction
1.1.1 Current Situation and Challenges of Food Safety and Regulations
Food can never be entirely safe. In recent years, food safety concern has grown significantly following a number of highly publicized incidents worldwide. These incidents include bovine spongiform encephalopathy in beef and benzene in carbonated drinks in the United Kingdom, dioxins in pork and milk products in Belgium, pesticides in contaminated foods in Japan, tainted coca-cola in Belgium and France, melamine in milk products in China, salmonella in peanuts and pistachios in the U.S. [1], and phthalates in drinks and foods in Taiwan [2]. Governments all over the world have taken many measures to increase food safety, resulting in a marked increase in the number of regulated compounds.
The European Union (EU) made a considerable effort to centralize food regulatory powers. The European Food Safety Authority (EFSA) and the national competent authorities are networks for food safety. The European Commission has designated food safety as a top priority, and published a white paper on food safety [3]. Legislative documents, such as 657/2002/EC, which sets out performance criteria for veterinary drug residue methods, are published as European Commission Decisions [4].
The Japanese government implemented a “positive list” to regulate the use of pesticides, veterinary drugs, and other chemicals in 2006, which replaced the old “negative list” regulations [5]. Over 700 compounds have to be monitored and reported. A certified safety report is now a requirement for both importing and exporting countries. The new regulations are listed as addendums to the positive list. In Japan, strengthening regulations for industrial use of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA), additives, and residual pharmaceutical and personal care products (PPCPs) in the environment is progressing, which in turn creates a demand for instrumentation that provides reliable trace determination.
In the United States, federal laws are the primary source of food safety regulations, for example, related codes under CFR Title 7, 9, 21, and 40. The law enforcement network comprises state government agencies and federal government agencies, including the U.S. Department of Agriculture (USDA), Food and Drug Administration (FDA), Centers for Disease Control and Prevention (CDC), and National Oceanic and Atmospheric Administration (NOAA). The Food Safety Modernization Act (H.R. 2751) is a federal statute signed into law by President Barack Obama on January 4, 2011. The law grants FDA authority to order recalls of contaminated food, increase inspections of domestic food facilities, and enhance detection of food-borne illness outbreaks.
As a result of regulation change and globalization, most nations around the world have now increased regulations on food safety for their domestic and export markets. International coordination and standardization are mainly conducted by the Codex Alimentarius Commission (CAC). The CAC is an intergovernmental body established in 1961 by the Food and Agriculture Organization of the United Nations (FAO), and joined by the World Health Organization (WHO) in 1962 to implement the Joint FAO/WHO Food Standards Program. There are 185 member countries and one organization member (EC) in the Codex now. The Codex standards are recommendations for voluntary application by members. However, in many cases, these standards are the basis for national legislation. The Codex covers processed, semiprocessed, and raw foods. The Codex also has general standards covering (but not limited to) food hygiene, food additives, food labeling, and pesticide residues [6].
1.1.2 Residues and Matrices of Food Analysis and High-Throughput Analysis
From the examples listed above, it is simply impossible to test every single item for every imaginable food-borne pathogen, including bacteria, viruses, and parasites; food allergens such as milk, eggs, shellfish, and soybean; naturally occurring toxins and mycotoxins; residues of pesticides and veterinary drugs; environmental contaminants; processing and packaging contaminants; spoilage markers [7]; food authenticity; and labeling accuracy [8].
Fortunately, modern analytical techniques, especially mass spectrometry-based techniques, such as gas chromatography–mass spectrometry (GC–MS) and liquid chromatography–mass spectrometry (LC–MS), can help speed up the processes. In the past decade, LC–MS, including tandem LC–MS techniques, or LC–MS/MS, has been applied in pesticide residue analysis and other food safety issues. The use of LC–MS has increased exponentially in recent years [9]. For example, an LC–MS/MS method using a scheduled selected reaction monitoring (sSRM) algorithm was developed and applied to analyze 242 multiclass pesticides for fruits and vegetables [10]. The high selectivity of LC–MS can effectively reduce interference from matrices, which significantly simplifies the process of sample preparation.
In addition, other high-throughput methods, including bioactivity-based methods, have also been widely applied today and will continue to be applied at least for the foreseeable future, although false-positive results were found in a high number of cases for these methods [11]. A striking example is the rapid microbiological assays used routinely by dairies to screen milk inexpensively and rapidly for residues of antimicrobial drugs. In the United Kingdom alone, dairy companies run millions of such assays per year, with a test duration of only minutes from sampling to result. These tests are widely used internationally by dairies for completeness.
1.1.3 Food Safety Classifications
Food safety analysis can be broadly classified and grouped based on the residues or analytes and food matrices, accepting that there will be some degree of crossover between groups. Based on the analytes, it can be classified to pesticide residues, drug residues, mycotoxins and environment pollutants, and other industrial chemicals. Based on food matrices, the most accepted classification of groups consists of high-moisture foods, low-moisture foods, and fatty foods. Examples of such matrices are fruits and vegetables, dry grains (wheat, rice, bean, etc.), and tissues, including fish and meat.
Food safety analysis methods can be further divided into two categories: screening methods and confirmation methods. The regulatory agencies and international standard organizations have clear guidelines for screening methods and confirmation methods. The requirements are slightly different for both, depending on the residues to be analyzed, matrix, risk factor, and techniques available. A screening method is qualitative or semiquantitative in nature, comprises establishment of those residues likely to be present based on an interpretation of the raw data, and tries to avoid false negatives as much as possible. A false negative rate of 5% is accepted for both the EU and the US FDA [12,13]. A confirmation method can provide unequivocal confirmation of the identity of the residue and may also confirm the quantity present on residues found in screening. Therefore, an analyst has to use appropriate guidelines to develop a new method based on the regulation, residue category, and matrices and to provide expert advice on the findings to those commissioning the analysis.
1.1.4 “High Throughput” Definition
The “high throughput” concept has become popular in the pharmaceutical industry after combinatorial chemistry was introduced for drug discovery [14], such as in “high-throughput screening” and “high-throughput drug analysis.” However, “high-throughput analysis for food safety” has only recently drawn more attention, especially after China's melamine milk crisis and Taiwan's phthalates scandal.
Although there is no numeric definition of “high-throughput screening” in the pharmaceutical industry, the standardized sample plate of 96-, 384-, or even 1536-well plates can indicate how quickly many analyses can be completed. Compared with single digits of targets in drug screening, food analysis often involves multiclass compounds ranging from a few dozens to a few hundred targets. All these kinds of GC–MS or LC–MS methods can be considered as high-throughput analyses because one way to calculate sample throughput is to use the following equation [15]:
where screening capacity or analysis capacity = number of target analytes that can be screened or analyzed by the method; total analysis time = time for sample preparation + instrument data acquisition + data analysis (data process) + documentation. Given this definition, analyses using GC–MS and LC–MS as already discussed can qualify as “high throughput” because their screening capacities can be, in some instances, quite high. High screening capacities eliminate the need for many analyses on the same sample that simply screen for just one or two analytes at a time. Practically, as long as the sample throughput of a new method is significantly higher than that obtained using the current prevailing method, the new method should be considered as a high-throughput method.