
eBook - ePub
Multicomponent Reactions
Concepts and Applications for Design and Synthesis
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Multicomponent Reactions
Concepts and Applications for Design and Synthesis
Book details
Book preview
Table of contents
Citations
About This Book
Addressing a dynamic aspect of organic chemistry, this book describes synthetic strategies and applications for multicomponent reactions – including key routes for synthesizing complex molecules. • Illustrates the crucial role and the important utility of multicomponent reactions (MCRs) to organic syntheses
• Compiles novel and efficient synthetic multicomponent procedures to give readers a complete picture of this class of organic reactions
• Helps readers to design efficient and practical transformations using multicomponent reaction strategies
• Describes reaction background, applications to synthesize complex molecules and drugs, and reaction mechanisms
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Multicomponent Reactions by Raquel P. Herrera, Eugenia Marqués-López in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Organic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
INTRODUCTION: MULTICOMPONENT STRATEGIES
Juan V. Alegre-Requena, Eugenia Marqués-López and Raquel P. Herrera
Departamento de Química Orgánica, Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC-Universidad de Zaragoza, Zaragoza, Spain
GENERAL INTRODUCTION
The goal of this book is to provide an overview of the most useful and noteworthy examples of multicomponent reactions (MCRs) published in this field between 2005 and 2014, in order to attract the attention of a wide range of readers. Previous examples are collected in an exceptional book edited by Zhu and Bienaymé, published in 2005 [1] . Since then, a great number of interesting and important reviews have also been written, and they will be cited throughout this book. For this reason, only the most pivotal examples will be reported and commented on in order to avoid repetitions.
MCRs are widely defined as reactions in which three or more components are added to a single vessel at the same time to lead to a final product that contains most of the atoms from the starting reagents. Therefore, these reactions encompass a sequence of more than one chemical transformation without the necessity of changing the reaction media after each transformation. It is not surprising then that MCRs lead to great molecular diversity and allow for the creation of libraries of small organic molecules while requiring less time and effort when compared with step-by-step procedures [2] . This is especially attractive for the pharmaceutical industry, for which the easy creation of large libraries of compounds with possible biological activity is a priority.
The significance of these processes can be observed in the large number of publications that have appeared in this field over the last decade. Also, the biological utility of compounds synthesized with MCRs has been confirmed by the discovery of many molecules with remarkable biological activity (Fig. 1.1) [7] .
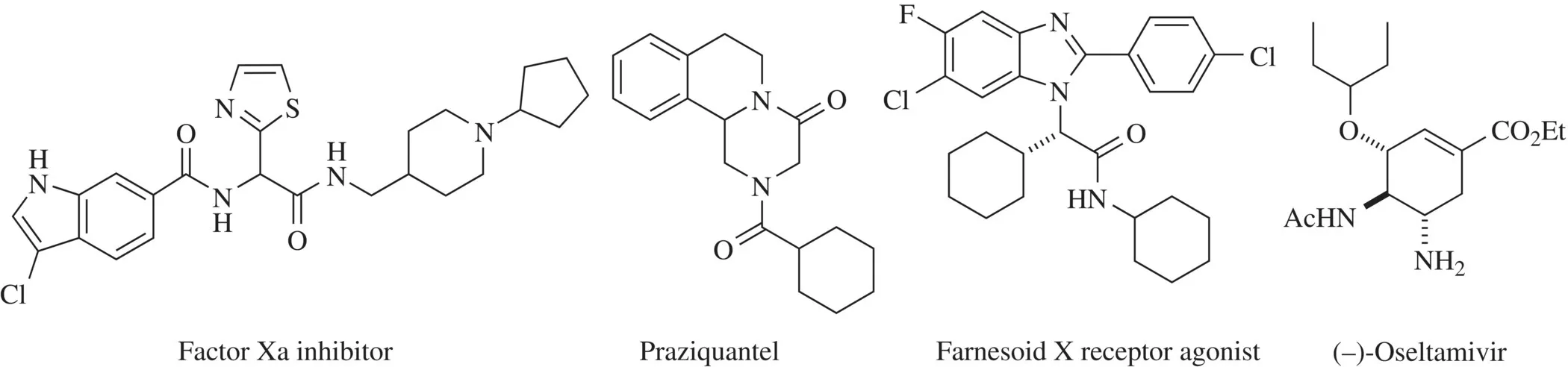
FIGURE 1.1 Examples of drugs synthesized with MCRs: factor Xa inhibitors [3] , praziquantel [4] , farnesoid X receptor agonists [5] , and (–)-oseltamivir [6] .
Over the last decade, interest in performing sustainable chemistry has drastically increased [8] . The application of ingenious strategies to synthesize complex scaffolds and highly substituted molecules, combining molecular diversity [9] with ecocompatibility [10] , has been the main focus of many scientific groups. In effect, the rational design of reactions that transform simple and readily available substrates into complex structures in a single reaction is one of the current major challenges in organic synthesis. In this context, MCRs have become one of the best established approaches for reaching this goal, since these strategies imply atom economy [11] and bond-forming efficiency [12] .
There are some authors that consider the reaction between bitter almond oil and ammonia, carried out by Laurent in 1838, as the first MCR [13] . This mixture could promote a condensation of ammonia, hydrogen cyanide, and benzaldehyde, resulting in an α-aminonitrile intermediate that, once formed, reacts with another molecule of benzaldehyde to give its corresponding Schiff base. However, in the compositions reported by the authors, none of the examined products lined up with the MCR’s possible products, neither the α-aminonitrile nor its subsequent Schiff base. Therefore, the Strecker reaction could be considered the first reported MCR, due to the fact that it was the first time that an author was able to determine the structure of a product formed in a MCR.
Since the development of the Strecker reaction in 1850 [14] , a great number of interesting MCRs have been reported, and amidst them, some of the most significant reactions are displayed in Table 1.1. In the following chapters, these pioneering reactions will be extensively discussed.
TABLE 1.1 Some historically significant MCRs
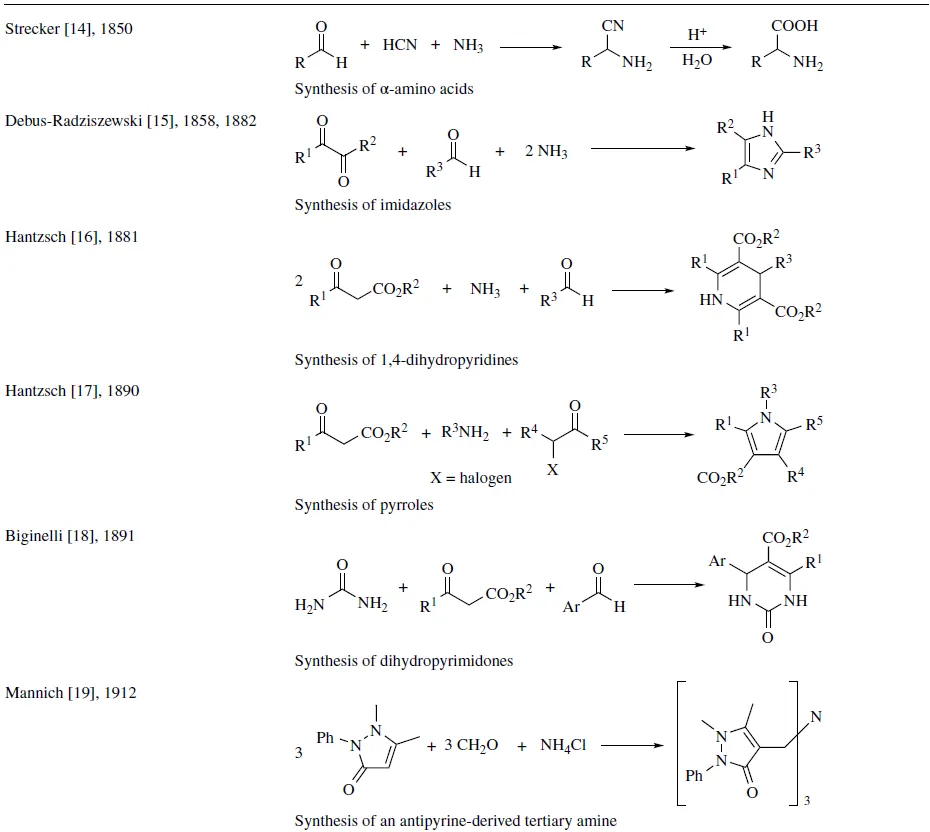
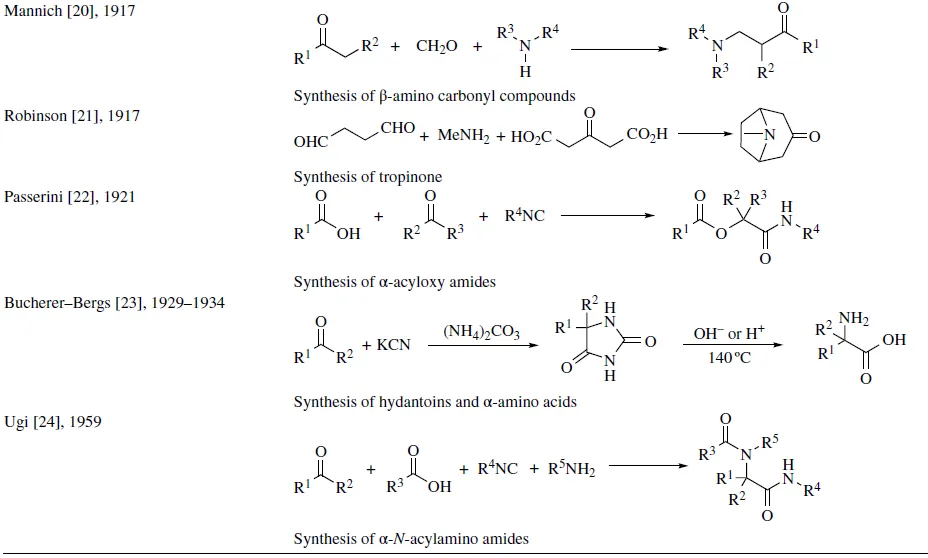
1.1 BASIC CONCEPTS
Some of the basic concepts related to MCRs are briefly described in the following text in order to familiarize the reader with this field and its characteristics.
1.1.1 Clarifying Terminology: One-Pot, Domino/Cascade, Tandem, and MCRs
The previous terms are probably familiar for most chemists, but they have crucial differences that are important to know in order to distinguish each term from the others. The term one-pot reaction includes reactions that involve multiple chemical transformations between reagents that are carried out in a single reactor. Thus, MCRs fall into the category of one-pot reactions due to the sole reactor required for carrying out the reaction and that there are multiple chemical transformations involved.
Furthermore, Fogg and dos Santos categorized the different types of multicatalyzed one-pot reactions in 2004 [25] , some years after Tietze set the definition of domino reactions [12] . In this categorization, domino/cascade catalysis, tandem catalysis, and multicatalytic one-pot reactions were distinguished depending on certain factors, such as the moment when the (pre)catalysts are added and the number of catalytic mechanisms involved (Fig. 1.2). Generally speaking, domino/cascade and tandem catalyses are one-pot reactions where all the components are introduced at the same time at the beginning of the reaction, while in multicatalytic one-pot reactions, all of the reaction’s components are not added at the same time. Another requirement for domino/cascade and tandem catalyses is that all successive transformations must occur as a consequence of the intermediate generated in the previous reaction step. In Fogg’s classification, domino/cascade and tandem catalyses are differentiated by the number of catalytic mechanisms present in the reaction.
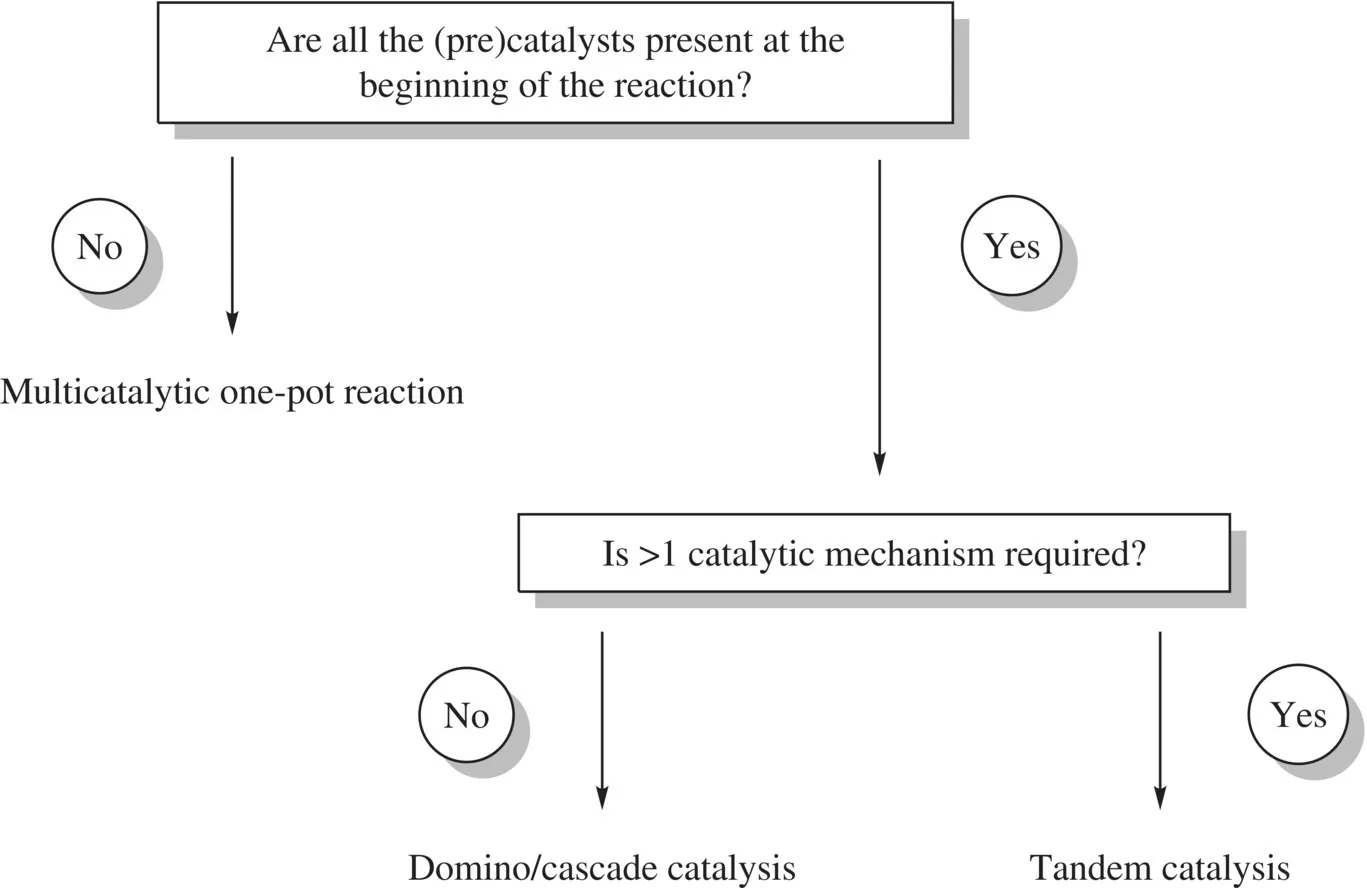
FIGURE 1.2 Fogg’s simple classification of one-pot processes involving multiple catalytic transformations.
With all the aforementioned concepts defined, it has been made clear that MCRs are one-pot reactions that might also fall under the category of domino/cascade or tandem reactions. A reaction is a domino/cascade or tandem MCR when it has the characteristics of one of these types of reactions in addition to incl...
Table of contents
- COVER
- TITLE PAGE
- TABLE OF CONTENTS
- LIST OF CONTRIBUTORS
- PREFACE
- LIST OF ABBREVIATIONS
- 1 INTRODUCTION
- 2 ORGANOCATALYTIC ASYMMETRIC MULTICOMPONENT REACTIONS
- 3 METAL-CATALYZED MULTICOMPONENT REACTIONS
- 4 MULTICOMPONENT REACTIONS WITH ORGANOBORON COMPOUNDS
- 5 CARBENE-PROMOTED MULTICOMPONENT REACTIONS
- 6 MULTICOMPONENT REACTIONS IN THE SYNTHESIS OF TARGET MOLECULES
- 7 RECENT ADVANCES IN THE UGI MULTICOMPONENT REACTIONS
- 8 PASSERINI MULTICOMPONENT REACTIONS
- 9 BIGINELLI MULTICOMPONENT REACTIONS
- 10 BUCHERER–BERGS AND STRECKER MULTICOMPONENT REACTIONS
- 11 UNUSUAL APPROACH FOR MULTICOMPONENT REACTIONS
- 12 ESSENTIAL MULTICOMPONENT REACTIONS I
- 13 ESSENTIAL MULTICOMPONENT REACTIONS II
- INDEX
- END USER LICENSE AGREEMENT