
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Computational Organic Chemistry
About this book
The Second Edition demonstrates how computational chemistry continues to shed new light on organic chemistry
The Second Edition of author Steven Bachrach's highly acclaimed Computational Organic Chemistry reflects the tremendous advances in computational methods since the publication of the First Edition, explaining how these advances have shaped our current understanding of organic chemistry. Readers familiar with the First Edition will discover new and revised material in all chapters, including new case studies and examples. There's also a new chapter dedicated to computational enzymology that demonstrates how principles of quantum mechanics applied to organic reactions can be extended to biological systems.
Computational Organic Chemistry covers a broad range of problems and challenges in organic chemistry where computational chemistry has played a significant role in developing new theories or where it has provided additional evidence to support experimentally derived insights. Readers do not have to be experts in quantum mechanics. The first chapter of the book introduces all of the major theoretical concepts and definitions of quantum mechanics followed by a chapter dedicated to computed spectral properties and structure identification. Next, the book covers:
- Fundamentals of organic chemistry
- Pericyclic reactions
- Diradicals and carbenes
- Organic reactions of anions
- Solution-phase organic chemistry
- Organic reaction dynamics
The final chapter offers new computational approaches to understand enzymes. The book features interviews with preeminent computational chemists, underscoring the role of collaboration in developing new science. Three of these interviews are new to this edition.
Readers interested in exploring individual topics in greater depth should turn to the book's ancillary website www.comporgchem.com, which offers updates and supporting information. Plus, every cited article that is available in electronic form is listed with a link to the article.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Quantum Mechanics for Organic Chemistry
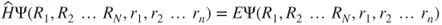
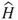
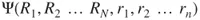
1.1 Approximations to the Schrödinger Equation—The Hartree–Fock Method
1.1.1 Nonrelativistic Mechanics
Table of contents
- Cover
- Title Page
- Copyright
- Dedication
- Preface
- Acknowledgments
- Chapter 1: Quantum Mechanics for Organic Chemistry
- Chapter 2: Computed Spectral Properties and Structure Identification
- Chapter 3: Fundamentals of Organic Chemistry
- Chapter 4: Pericyclic Reactions
- Chapter 5: Diradicals and Carbenes
- Chapter 6: Organic Reactions of Anions
- Chapter 7: Solution-Phase Organic Chemistry
- Chapter 8: Organic Reaction Dynamics
- Chapter 9: Computational Approaches to Understanding Enzymes
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app