
eBook - ePub
Calorimetry
Fundamentals, Instrumentation and Applications
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Calorimetry
Fundamentals, Instrumentation and Applications
Book details
Book preview
Table of contents
Citations
About This Book
Clearly divided into three parts, this practical book begins by dealing with all fundamental aspects of calorimetry. The second part looks at the equipment used and new developments. The third and final section provides measurement guidelines in order to obtain the best results.
The result is optimized knowledge for users of this technique, supplemented with practical tips and tricks.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Calorimetry by Stefan Mathias Sarge,Günther W. H. Höhne,Wolfgang Hemminger in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Analytic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Part One
Fundamentals of Calorimetry
1
Methods of Calorimetry
This chapter provides a brief outline of the principles of heat measurement. A classification scheme will be developed on the basis of simple examples. A more detailed treatment of the procedures and calorimeters involved can be found in the second part of the book.
1.1 Compensation of the Thermal Effect
The heat released from a sample during a process flows into the calorimeter and would cause a temperature change of the latter as a measuring effect; this thermal effect is continuously suppressed by compensating the respective heat flow. The methods of compensation include the use of “latent heat” caused by a phase transition, thermoelectric effects, heats of chemical reactions, a change in the pressure of an ideal gas (Ter Minassian and Milliou, 1983), and heat exchange with a liquid1) (Regenass, 1977). Because the last three methods are confined to special cases, only the compensation by a physical heat of transition and by electric effects are briefly discussed here.
1.1.1 Compensation by a Phase Transition
Around 1760, Black2) (Robison, 1803) realized that the heat delivered to melting ice serves for a transition from the solid to the liquid state at a constant temperature. Indeed, although the melting of ice requires a steady supply of heat, the temperature of the ice–water mixture only begins to rise after all the ice has melted. Black is said to have been the first to have used this “latent heat of fusion” of ice for the measurement of heat. His “phase transition calorimeter” was very simple. He placed a warm sample in a cavity inside a block of ice and sealed the cavity with an ice sheet. After the sample had assumed the temperature of ice, he determined the mass of the melted ice by weighing.
The principle of this method is that the heat ΔQ exchanged with the calorimeter is not measured as a heat flow but causes a phase transition in a suitable substance (e.g., ice). If the specific heat of transition qtrs of the respective substance is known, the heat involved can be determined because it is proportional to the mass of the transformed substance Δm:
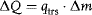
The mass of the transformed substance Δm is determined either directly by weighing or indirectly (e.g., by measuring the volume change due to the difference between the densities of the two phases).
The first usable calorimeter involving a phase transition – the “ice calorimeter”– was developed by Lavoisier and Laplace (1780). Figure 1.1 schematically shows the design of this device. The sample chamber is completely surrounded by a double-walled vessel containing pieces of ice. This inner ice jacket is surrounded by a second double-walled vessel filled with an ice–water mixture (outer ice jacket). The whole system is in thermal equilibrium at 0 °C. The basic idea in this calorimeter is that the measuring system proper (i.e., the inner ice jacket) is insulated by the outer jacket, in which any disturbing influence of heat from the environment on the inner ice jacket is compensated by an ice–water phase transition in the outer jacket. Only heat released inside the sample chamber serves for the melting of ice in the inner ice jacket. Because there is no temperature difference between the inner and outer jackets, no heat exchange between them takes place. Lavoisier and Laplace designated the measured heat as the “mass of melted ice.” The specific heat capacities of solids and liquids, as well as heats of combustion and the production of heat by animals, were measured this way. These measurements were carried out in winter when low environmental temperatures allowed experiments to be carried out over longer periods of time (up to 20 h) in order to measure relatively small heat from animals, for example. The ice calorimeter of Lavoisier and Laplace is very bulky; moreover, the inner ice jacket must be carefully prepared before each test. In addition, it suffers from a systematic error that stems from the influx of relatively warm air at the lid. This air cools down in the calorimeter, thus releasing heat, and escapes with the downward flow of ice water. Another systematic error that affects the accuracy results from the fact that the water layer located in the inner jacket between the pieces of ice may attain a local temperature of several degree C, depending on the magnitude of the heat and the rate of its release. These layers may rise because of density differences (Figure 1.2) and transfer part of...
Table of contents
- Cover
- Table of Contents
- Related Titles
- Title
- Authors
- Copyright
- Preface
- List of Quantities and Units
- Introduction: Calorimetry: Definition, Application Fields and Units
- Part One: Fundamentals of Calorimetry
- Part Two: Practice of Calorimetry
- Index
- End User License Agreement