
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Molecular Rearrangements in Organic Synthesis
Book details
Book preview
Table of contents
Citations
About This Book
Designed for practitioners of organic synthesis, this book helps chemists understand and take advantage of rearrangement reactions to enhance the synthesis of useful chemical compounds.
- Provides ready access to the genesis, mechanisms, and synthetic utility of rearrangement reactions
- Emphasizes strategic synthetic planning and implementation
- Covers 20 different rearrangement reactions
- Includes applications for synthesizing compounds useful as natural products, medicinal compounds, functional materials, and physical organic chemistry
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Molecular Rearrangements in Organic Synthesis by Christian M. Rojas in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Organic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
PART I
1,2-MIGRATIONS
CHAPTER 1
PINACOL AND SEMIPINACOL REARRANGEMENTS IN TOTAL SYNTHESIS
Alison Xiang Gao
Department of Chemistry, The Scripps Research Institute, Jupiter, FL, USA
Stephen B. Thomas
Department of Chemistry, Columbia University, NY, USA
Scott A. Snyder
Department of Chemistry, The Scripps Research Institute, Jupiter, FL, USA; Department of Chemistry, Columbia University, NY, USA
1.1 INTRODUCTION
Among the array of reactions available to alter molecular complexity, pinacol and semipinacol rearrangements have a particularly long history, constituting among the very first (if not the first) rearrangement reactions discovered by synthetic chemists.1 However, despite being known for over a century and a half, their use in complex natural product synthesis has only recently come of age. Indeed, with a clearer understanding of the factors governing their regio- and stereoselectivity, as well as more powerful variants (including asymmetric) that can induce the rearrangement under mild conditions, these processes have a number of specific, but highly valuable, applications whose wealth is beginning to be tapped with ever greater frequency. This chapter seeks to provide a sense of the current state of the art of both pinacol and semipinacol processes, discussing each separately under the rubric of recent applications.
1.2 PINACOL REACTION
1.2.1 Background and Introduction
We begin with the pinacol rearrangement, a reaction process whose name derives from the starting material used in the earliest known example of the transformation. That event, the exposure of pinacol (1, 2,3-dimethylbutane-2,3-diol; Scheme 1.1) to sulfuric acid, produced pinacolone (2, 3,3-dimethylbutane-2-one). Although this reaction was first performed by Fittig in 1860,2 it was not until the early 1870s that the actual structure of the product was confirmed by Butlerov3; this lapse not only reflects the challenges of determining structure in that era but also the fact that rearrangements were effectively unknown. In fact, a contemporary publication by Kekulé (his seminal paper on representing organic structures) included rules which suggested that carbon skeletal rearrangements could not occur.4 In any event, by the end of the 19th century, the overall process depicted for the conversion of 1 to 2 was clear in terms of starting material and product. As additional substrates proved amenable to the process, the term pinacol rearrangement has since been used more broadly to define the conversion of any acyclic or cyclic vicinal diol into an aldehyde or ketone under acidic (proton or Lewis) conditions.5
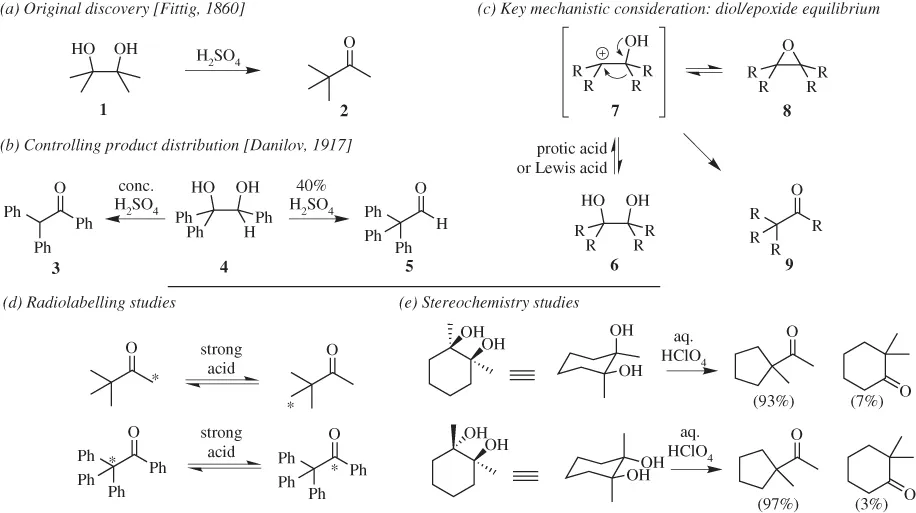
Scheme 1.1 The pinacol rearrangement: discovery and key considerations.
Critically, as with many other skeletal rearrangements, there are subtleties that define both its mechanism as well as the products that can be generated from a given substrate under a specific set of reaction conditions. One early clue to that complexity derived from the observation by Danilov in 1917 that a single diol substrate (4) could yield different carbonyl-containing products (3 or 5) based solely on the strength of the acid deployed (Scheme 1.1b).6 Although these outcomes reflect kinetic control, that analysis, in and of itself, is insufficient given that separate study has shown that both 4 and 5 can interconvert, suggesting the prospect of reversibility as part of the pinacol rearrangement process itself. Indeed, that concept was beautifully illustrated by Fry in a subsequent series of elegant 14C-labeling studies which showed the transposition of carbon atoms of an array of pinacol “products” exposed to strongly acidic conditions (Scheme 1.1d).7 A second critical observation resides in the fact that a number of pinacol rearrangements produce epoxides in addition to the standard carbonyl-containing adduct. In some cases, these materials can be induced to rearrange to standard pinacol products, while in others, they are inert to the reaction conditions. Finally, there is a wide range of contexts, employing both protic acids and Lewis acids under varying reaction conditions, that can induce the rearrangement to occur for most individual substrates (with expected variations in yield).
Collectively, these findings indicate that very few mechanistic conclu...
Table of contents
- Cover
- Title Page
- Copyright
- Table of Contents
- LIST OF CONTRIBUTORS
- PREFACE
- PART I: 1,2-MIGRATIONS
- PART II: 1,2-MIGRATIONS VIA THREE-MEMBERED RINGS
- PART III: 1,3-TRANSPOSITIONS
- Part IV: [3,3]- AND [2,3]-SIGMATROPIC REARRANGEMENTS
- PART V: IPSO REARRANGEMENTS
- INDEX
- End User License Agreement