
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Now in it's 3rd Edition, Industrial Catalysis offers all relevant information on catalytic processes in industry, including many recent examples. Perfectly suited for self-study, it is the ideal companion for scientists who want to get into the field or refresh existing knowledge.
The updated edition covers the full range of industrial aspects, from catalyst development and testing to process examples and catalyst recycling. The book is characterized by its practical relevance, expressed by a selection of over 40 examples of catalytic processes in industry. In addition, new chapters on catalytic processes with renewable materials and polymerization catalysis have been included. Existing chapters have been carefully revised and supported by new subchapters, for example, on metathesis reactions, refinery processes, petrochemistry and new reactor concepts.
"I found the book accesible, readable and interesting - both as a refresher and as an introduction to new topics - and a convenient first reference on current industrial catalytic practise and processes."
Excerpt from a book review for the second edition by P. C. H. Mitchell, Applied Organometallic Chemistry (2007)
The updated edition covers the full range of industrial aspects, from catalyst development and testing to process examples and catalyst recycling. The book is characterized by its practical relevance, expressed by a selection of over 40 examples of catalytic processes in industry. In addition, new chapters on catalytic processes with renewable materials and polymerization catalysis have been included. Existing chapters have been carefully revised and supported by new subchapters, for example, on metathesis reactions, refinery processes, petrochemistry and new reactor concepts.
"I found the book accesible, readable and interesting - both as a refresher and as an introduction to new topics - and a convenient first reference on current industrial catalytic practise and processes."
Excerpt from a book review for the second edition by P. C. H. Mitchell, Applied Organometallic Chemistry (2007)
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Introduction
1.1 The Phenomenon Catalysis
Catalysis is the key to chemical transformations. Most industrial syntheses and nearly all biological reactions require catalysts. Furthermore, catalysis is the most important technology in environmental protection, that is, the prevention of emissions. A well-known example is the catalytic converter for automobiles.
Catalytic reactions were already used in antiquity, although the underlying principle of catalysis was not recognized at the time. For example, the fermentation of sugar to ethanol and the conversion of ethanol to acetic acid are catalyzed by enzymes (biocatalysts). However, the systematic scientific development of catalysis only began about 200 years ago, and its importance has grown up to the present day [1].
The term “catalysis” was introduced as early as 1836 by Berzelius in order to explain various decomposition and transformation reactions. He assumed that catalysts possess special powers that can influence the affinity of chemical substances.
A definition that is still valid today is due to Ostwald (1895): “a catalyst accelerates a chemical reaction without affecting the position of the equilibrium.” Ostwald recognized catalysis as a ubiquitous phenomenon that was to be explained in terms of the laws of physical chemistry.
While it was formerly assumed that the catalyst remained unchanged in the course of the reaction, it is now known that the catalyst is involved in chemical bonding with the reactants during the catalytic process. Thus, catalysis is a cyclic process: the reactants are bound to one form of the catalyst, and the products are released from another, regenerating the initial state.
In simple terms, the catalytic cycle can be described as shown in Figure 1.1. The intermediate catalyst complexes are in most cases highly reactive and difficult to detect.
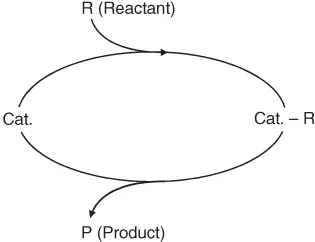
Figure 1.1 Catalytic cycle.
In theory, an ideal catalyst would not be consumed, but this is not the case in practice. Owing to competing reactions, the catalyst undergoes chemical changes, and its activity becomes lower (catalyst deactivation). Thus, catalysts must be regenerated or eventually replaced.
Apart from accelerating reactions, catalysts have another important property: they can influence the selectivity of chemical reactions. This means that completely different products can be obtained from a given starting material by using different catalyst systems. Industrially, this targeted reaction control is often even more important than the catalytic activity.
Catalysts can be gases, liquids, or solids. Most industrial catalysts are liquids or solids, whereby the latter react only via their surface. The importance of catalysis in the chemical industry is shown by the fact that 75% of all chemicals are produced with the...
Table of contents
- Cover
- Related Titles
- Title Page
- Copyright
- Table of Contents
- Preface to the Third Edition
- Abbreviations
- Chapter 1: Introduction
- Chapter 2: Homogeneous Catalysis with Transition Metal Catalysts
- Chapter 3: Homogeneously Catalyzed Industrial Processes
- Chapter 4: Biocatalysis
- Chapter 5: Heterogeneous Catalysis: Fundamentals
- Chapter 6: Catalyst Shapes and Production of Heterogeneous Catalysts
- Chapter 7: Shape-Selective Catalysis: Zeolites
- Chapter 8: Heterogeneously Catalyzed Processes in Industry
- Chapter 9: Refinery Processes and Petrochemistry
- Chapter 10: Electrocatalytic Processes
- Chapter 11: Environmental Catalysis and Green Chemistry
- Chapter 12: Phase-Transfer Catalysis
- Chapter 13: Catalytic Processes with Renewable Materials
- Chapter 14: Polymerization Catalysis
- Chapter 15: Planning, Development, and Testing of Catalysts
- Chapter 16: Catalysis Reactors
- Chapter 17: Economic Importance of Catalysts
- Chapter 18: Future Development of Catalysis
- Solutions to the Exercises
- Index
- End User License Agreement
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Industrial Catalysis by Jens Hagen in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Chemistry. We have over one million books available in our catalogue for you to explore.