
Understanding Organometallic Reaction Mechanisms and Catalysis
Computational and Experimental Tools
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Understanding Organometallic Reaction Mechanisms and Catalysis
Computational and Experimental Tools
About This Book
Exploring and highlighting the new horizons in the studies of reaction mechanisms that open joint application of experimental studies and theoretical calculations is the goal of this book. The latest insights and developments in the mechanistic studies of organometallic reactions and catalytic processes are presented and reviewed. The book adopts a unique approach, exemplifying how to use experiments, spectroscopy measurements, and computational methods to reveal reaction pathways and molecular structures of catalysts, rather than concentrating solely on one discipline. The result is a deeper understanding of the underlying reaction mechanism and correlation between molecular structure and reactivity. The contributions represent a wealth of first-hand information from renowned experts working in these disciplines, covering such topics as activation of small molecules, C-C and C-Heteroatom bonds formation, cross-coupling reactions, carbon dioxide converison, homogeneous and heterogeneous transition metal catalysis and metal-graphene systems. With the knowledge gained, the reader will be able to improve existing reaction protocols and rationally design more efficient catalysts or selective reactions. An indispensable source of information for synthetic, analytical, and theoretical chemists in academia and industry.
Frequently asked questions
Information
Chapter 1
Mechanisms of Metal-Mediated C–N Coupling Processes: A Synergistic Relationship between Gas-Phase Experiments and Computational Chemistry
1.1 Introduction
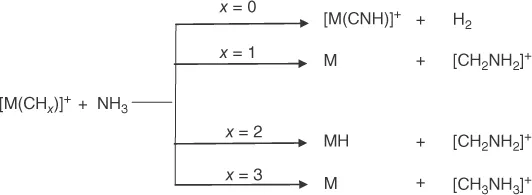
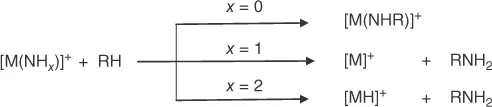
1.2 From Metal-Carbon to Carbon–Nitrogen Bonds
1.2.1 Thermal Reactions of Metal Carbide and Metal Methylidene Complexes with Ammonia
Table of contents
- Cover
- Related Titles
- Title Page
- Copyright
- List of Contributors
- Preface
- Chapter 1: Mechanisms of Metal-Mediated C–N Coupling Processes: A Synergistic Relationship between Gas-Phase Experiments and Computational Chemistry
- Chapter 2: Fundamental Aspects of the Metal-Catalyzed C–H Bond Functionalization by Diazocarbenes: Guiding Principles for Design of Catalyst with Non-redox-Active Metal (Such as Ca) and Non-Innocent Ligand
- Chapter 3: Using Metal Vinylidene Complexes to Probe the Partnership Between Theory and Experiment
- Chapter 4: Ligand, Additive, and Solvent Effects in Palladium Catalysis – Mechanistic Studies En Route to Catalyst Design
- Chapter 5: Computational Studies on Sigmatropic Rearrangements via π-Activation by Palladium and Gold Catalysts
- Chapter 6: Theoretical Insights into Transition Metal-Catalyzed Reactions of Carbon Dioxide
- Chapter 7: Catalytically Enhanced NMR of Heterogeneously Catalyzed Hydrogenations
- Chapter 8: Combined Use of Both Experimental and Theoretical Methods in the Exploration of Reaction Mechanisms in Catalysis by Transition Metals
- Chapter 9: Is There Something New Under the Sun?Myths and Facts in the Analysis of Catalytic Cycles
- Chapter 10: Computational Tools for Structure, Spectroscopy and Thermochemistry
- Chapter 11: Computational Modeling of Graphene Systems Containing Transition Metal Atoms and Clusters
- Index
- End User License Agreement