
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Intermediate Organic Chemistry
Book details
Book preview
Table of contents
Citations
About This Book
This book presents key aspects of organic synthesis – stereochemistry, functional group transformations, bond formation, synthesis planning, mechanisms, and spectroscopy – and a guide to literature searching in a reader-friendly manner. • Helps students understand the skills and basics they need to move from introductory to graduate organic chemistry classes
• Balances synthetic and physical organic chemistry in a way accessible to students
• Features extensive end-of-chapter problems
• Updates include new examples and discussion of online resources now common for literature searches
• Adds sections on protecting groups and green chemistry along with a rewritten chapter surveying organic spectroscopy
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Intermediate Organic Chemistry by Ann M. Fabirkiewicz, John C. Stowell in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Organic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
READING NOMENCLATURE
Organic chemistry is understood in terms of molecular structures as represented pictorially. Cataloging, writing, and speaking about these structures require a nomenclature system, the basics of which you have studied in your introductory course. To go further with the subject, you must begin reading journals, and this requires understanding of the nomenclature of complex molecules. This chapter presents a selection of compounds to illustrate the translation of names to structural representations. The more difficult task of naming complex structures is not covered here because each person’s needs will be specialized and can be found in nomenclature guides [1–5]. Most of the nomenclature rules are used to eliminate alternative names and arrive at a unique (or nearly so) name for a particular structure; thus, when beginning with names, you will need to know only a small selection of the rules in order to simply read the names and provide a structure. Although the subject of nomenclature is vast, these selections will enable you to understand many names in current journals.
1.1 ACYCLIC POLYFUNCTIONAL MOLECULES
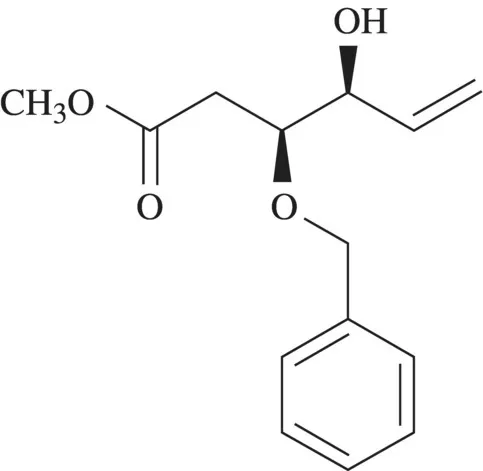
The space after methyl and the “ate” ending tells you this is a methyl ester. The acid from which the ester derives is a six-carbon chain with a double bond between carbons 5 and 6. There is an alcohol function on carbon 4. There is a methoxy group on carbon 3 and a phenyl group on the carbon of the methoxy group. Carbons 3 and 4 are stereogenic atoms each with S configuration as designated.
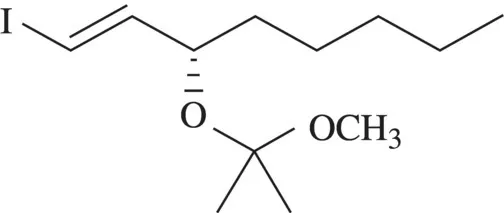
This is an example of a derivative name, that is, the first word is the complete name of an alcohol and the other two words describe a derivatization where the alcohol is converted to an ether (ketal). Such a name would be useful in discussing a compound that has the ketal present as a temporary entity, for example, as a protecting group.
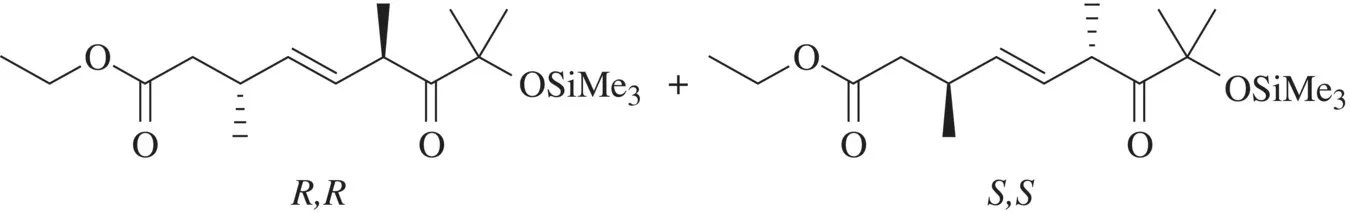
This is the ethyl ester of a nine-carbon unsaturated acid with substituents. The oxo indicates that there is a keto function on carbon 7. Be careful to distinguish this from the prefix oxa-, which has a different meaning; see Section 1.6. The asterisks indicate that the configuration designation is not absolute but rather represents that stereoisomer and/or the enantiomer thereof. Thus this name represents the R,R and/or the S,S isomers, but not R,S or S,R. This designation excludes diastereomers and is a common way to indicate a racemate.
1.2 MONOCYCLIC ALIPHATIC COMPOUNDS
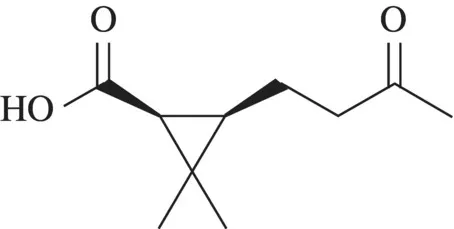
The ring is placed in the plane of the paper. Numbering of the ring starts at the location of the highest priority substitution, the carboxylic acid in this case. The butyl substituent on the third carbon of the ring has a keto ...
Table of contents
- COVER
- TITLE PAGE
- TABLE OF CONTENTS
- PREFACE TO THE THIRD EDITION
- PREFACE TO THE SECOND EDITION
- PREFACE TO THE FIRST EDITION
- 1 READING NOMENCLATURE
- 2 ACCESSING CHEMICAL INFORMATION
- 3 STEREOCHEMISTRY
- 4 MECHANISMS AND PREDICTIONS
- 5 ELECTRON DELOCALIZATION, AROMATIC CHARACTER, AND PERICYCLIC REACTIONS
- 6 FUNCTIONAL GROUP TRANSFORMATIONS
- 7 CARBON–CARBON BOND FORMATION
- 8 PLANNING MULTISTEP SYNTHESES
- 9 PHYSICAL INFLUENCES ON REACTIONS
- 10 SURVEY OF ORGANIC SPECTROSCOPY
- APPENDIX A: COMMON CHEMICAL ABBREVIATIONS
- APPENDIX B: COMMON PROTECTING GROUPS
- INDEX
- END USER LICENSE AGREEMENT