
Inorganic Hydrazine Derivatives
Synthesis, Properties and Applications
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Inorganic Hydrazine Derivatives
Synthesis, Properties and Applications
About This Book
Traditionally, interest in the chemistry of hydrazine and its derivatives has been focused on the development of propellants and explosives, but in recent years a wide variety of new applications have emerged in fields such as polymers, pharmaceuticals, water treatment, agriculture and medicine. Inorganic Hydrazine Derivatives: Synthesis, Properties and Applications presents a comprehensive review of the research carried out in this field during the last four decades.
Methods for synthesizing inorganic hydrazine derivatives and complexes are systematically presented, together with details of their characterization, spectra, thermal analysis, crystal structure, and applications. Strong emphasis is given to controlling the reactivity of hydrazine derivatives from detonation to deflagration to decomposition. The monograph also highlights current developments and applications of inorganic hydrazine derivatives, including the synthesis of nanostructured materials.
Topics covered include:
- An introduction to hydrazine and its inorganic derivatives
- Hydrazine salts
- Metal hydrazines
- Metal hydrazine carboxylates
- Hydrazinium metal complexes
- Applications of inorganic hydrazine derivatives
This applications-based handbook is a valuable resource for academics and industry professionals researching and developing hydrazine compounds, high energy materials, nanomaterials, and pharmaceuticals.
Frequently asked questions
Information
1.1 Introduction

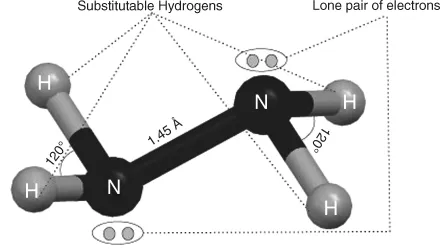
1.1.1 Properties of Hydrazine
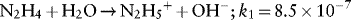
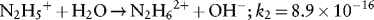
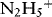
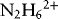
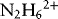
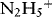
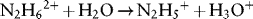
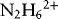
1.1.1.1 Redox Properties
Table of contents
- Cover
- Title Page
- Copyright
- Dedication
- List of Contributors
- Foreword
- Preface
- Acknowledgements
- Chapter 1: Hydrazine and Its Inorganic Derivatives
- Chapter 2: Hydrazine Salts
- Chapter 3: Metal Hydrazines
- Chapter 4: Metal Hydrazine Carboxylates
- Chapter 5: Hydrazinium Metal Complexes
- Chapter 6: Applications of Inorganic Hydrazine Derivatives
- Index