
Corrosion and Materials Selection
A Guide for the Chemical and Petroleum Industries
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About This Book
The petroleum and chemical industries contain a wide variety of corrosive environments, many of which
are unique to these industries. Oil and gas production operations consume a tremendous amount of iron
and steel pipe, tubing, pumps, valves, and sucker rods. Metallic corrosion is costly. However, the cost of
corrosion is not just financial. Beyond the huge direct outlay of funds to repair or replace corroded structures are the indirect costs – natural resources, potential hazards, and lost opportunity. Wasting natural resources is a direct contradiction to the growing need for sustainable development.
By selecting the correct material and applying proper corrosion protection methods, these costs can be
reduced, or even eliminated. This book provides a minimum design requirement for consideration when
designing systems in order to prevent or control corrosion damage safely and economically, and addresses:
•Corrosion problems in petroleum and chemical industries
•Requirements for corrosion control
•Chemical control of corrosive environments
•Corrosion inhibitors in refineries and petrochemical plants
•Materials selection and service life of materials
•Surface preparation, protection and maintainability
•Corrosion monitoring - plant inspection techniques and laboratory corrosion testing techniques
Intended for engineers and industry personnel working in the petroleum and chemical industries, this book is also a valuable resource for research and development teams, safety engineers, corrosion specialists and researchers in chemical engineering, engineering and materials science.
Frequently asked questions
Information
Chapter 1
Fundamentals of Corrosion in the Oil, Gas, and Chemical Industries
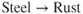
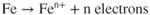
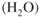
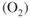
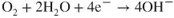
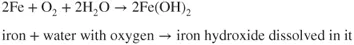
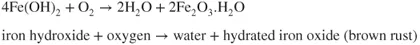
- Ions are involved and need a medium to move in (usually water).
- Oxygen is involved and needs to be supplied.
- The metal has to be willing to give up electrons to start the process.
- A new material is formed and this may react again or could protect the original metal.
- A series of simple steps are involved and a driving force is needed to achieve them.
- The most important fact is that interfering with the steps allows the corrosion reaction to be stopped or slowed to a manageable rate.
1.1 Uniform Corrosion
- Slow down or stop the movement of electrons:
- Coat the surface with a non-conducting medium such as paint, lacquer or oil
- Reduce the conductivity of the solution in contact with the metal, an extreme case being to keep it dry
- Wash away conductive pollutants regularly
- Apply a current to the material (see cathodic protection).
- Slow down or stop oxygen from reaching the surface. This is difficult to do completely, but coatings can help.
- Prevent the metal from giving up electrons:
- Use a more corrosion-resistant metal higher in the electrochemical series,
- Use a sacrificial coating that gives up its electrons more easily than the metal being protected
- Apply cathodic protection
- Use inhibitors.
- Select a metal that forms an oxide that is protective and stops the reaction.
- Control and consideration of environmental and thermal factors is also essential.
1.2 Localized Corrosion
1.2.1 Galvanic Corrosion
- The metals need to be in contact electrically.
- One metal needs to be significantly better at giving up electrons than the other
- An additional path for ion and electron movement is necessary.
- Break the electrical contact using plastic insulators or coatings between the metals.
- Select metals close together in the galvanic series.
- Prevent ion movement by coating the junction with an impermeable material, or ensure the environment is dry and that liquids cannot be trapped.
1.2.2 Pitting Corrosion
- selecting a resistant material,
- ensuring a high enough flow velocity of fluids in contact with the material or
- frequent washing,
- control of th...
Table of contents
- Cover
- Title Page
- Copyright
- Dedication
- About the Author
- Preface
- Acknowledgements
- Chapter 1: Fundamentals of Corrosion in the Oil, Gas, and Chemical Industries
- Chapter 2: Corrosion Problems in the Petroleum and Chemical Industries
- Chapter 3: Corrosion Considerations in Material Selection
- Chapter 4: Engineering Materials
- Chapter 5: Chemical Control of Corrosive Environments
- Chapter 6: Requirements for Corrosion Control in the Petroleum and Petrochemical Industries
- Chapter 7: Corrosion Inhibitors in Refineries and Petrochemical Plants
- Chapter 8: Corrosion Inhibitor Evaluations
- Chapter 9: Compatibility in Material Selection
- Chapter 10: Surface Preparation, Protection and Maintenance
- Chapter 11: Fabrication and Choice of Material to Minimize Corrosion Damage
- Chapter 12: Heat Treatment
- Glossary of Terms
- Bibliography
- Index
- End User License Agreement