
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Keynotes in Organic Chemistry
About this book
KEYNOTES IN Organic Chemistry
SECOND EDITION
This concise and accessible textbook provides notes for students studying chemistry and related courses at undergraduate level, covering core organic chemistry in a format ideal for learning and rapid revision. The material, with an emphasis on pictorial presentation, is organised to provide an overview of the essentials of functional group chemistry and reactivity, leading the student to a solid understanding of the basics of organic chemistry.
This revised and updated second edition of Keynotes in Organic Chemistry includes:
- new margin notes to emphasise links between different topics,
- colour diagrams to clarify aspects of reaction mechanisms and illustrate key points, and
- a new keyword glossary.
In addition, the structured presentation provides an invaluable framework to facilitate the rapid learning, understanding and recall of critical concepts, facts and definitions. Worked examples and questions are included at the end of each chapter to test the reader's understanding.
Reviews of the First Edition
" …this text provides an outline of what should be known and understood, including fundamental concepts and mechanisms."
Journal of Chemical Education, 2004
" Despite the book's small size, each chapter is thorough, with coverage of all important reactions found at first-year level... ideal for the first-year student wishing to revise… and priced and designed appropriately."
The Times Higher Education Supplement, 2004
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information

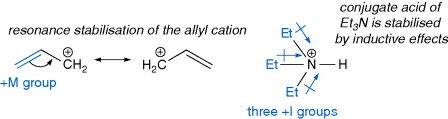
1.1 Ionic versus Covalent Bonds
- Ionic bonds are formed between molecules with opposite charges. The negatively charged anion will electrostatically attract the positively charged cation. This is present in (inorganic) salts.

- Covalent bonds are formed when a pair of electrons is shared between two atoms. A single line represents the two-electron bond.
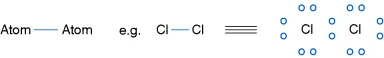
- Coordinate (or dative) bonds are formed when a pair of electrons is shared between two atoms. One atom donates both electrons and a single line or an arrow represents the two-electron bond.

- Hydrogen bonds are formed when the partially positive (δ+) hydrogen of one molecule interacts with the partially negative (δ−) heteroatom (e.g. oxygen or nitrogen) of another molecule.

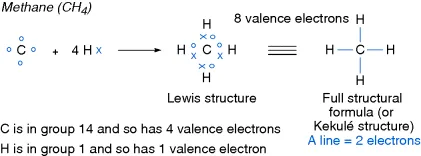
1.2 The Octet Rule

1.3 Formal Charge
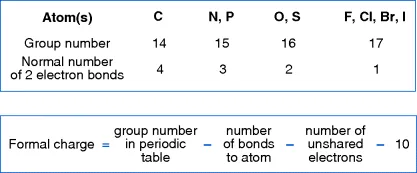

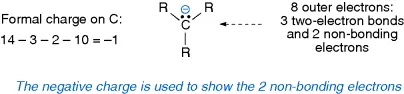
- Carbanions –three covalent bonds to carbon and a formal negative charge.
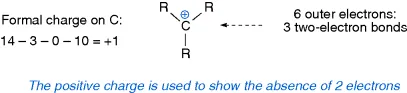
- Carbocations –three covalent bonds to carbon and a formal positive charge.
1.4 Sigma (σ–) and pi (π–) Bonds
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- Chapter 1: Structure and Bonding
- Chapter 2: Functional Groups, Nomenclature and Drawing Organic Compounds
- Chapter 3: Stereochemistry
- Chapter 4: Reactivity and Mechanism
- Chapter 5: Halogenoalkanes
- Chapter 6: Alkenes and Alkynes
- Chapter 7: Benzenes
- Chapter 8: Carbonyl Compounds: Aldehydes and Ketones
- Chapter 9: Carbonyl Compounds: Carboxylic Acids and Derivatives
- Chapter 10: Spectroscopy
- Chapter 11: Natural Products and Synthetic Polymers
- Appendix 1: Bond Dissociation Enthalpies
- Appendix 2: Bond Lengths
- Appendix 3: Approximate pKa Values (Relative to Water)
- Appendix 4: Useful Abbreviations
- Appendix 5: Infrared Absorptions
- Appendix 6: Approximate NMR Chemical Shifts
- Appendix 7: Reaction Summaries
- Appendix 8: Glossary
- Further Reading
- Outline Answers
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app