![]()
Chapter 1
Introduction
Jie Jack Li
1.1 Nomenclature of Heterocycles
What’s in a name? That which we call rose by any other name would smell as sweet. [William Shakespeare, Romeo and Juliet (II, ii, 1–2)].
Contrary to Shakespeare’s exclamation, naming heterocycles is an integral part of our learning of heterocyclic chemistry. They are the professional jargon that we routinely use to communicate with our peers.
Heterocycles, as the name suggests, are cyclic compounds containing one or more heteroatoms such as N, O, S, P, Si, B, Se, and Se. They may be further divided into aromatic heterocycles and saturated heterocycles. This book will focus largely on aromatic heterocycles. Saturated heterocycles represent a smaller portion of drugs. Another way of naming heterocycles is using the size of the heterocyclic rings. Therefore, they may be classified as three-, four-, five-, six-, and seven-membered heterocycles, and so on.
Three-membered heterocycles are important reaction intermediates in organic chemistry and in preparing medicines. But they usually do not exist in final drugs because they are reactive in physiological environments. Exceptions are found in cancer drugs such as epothilone A and mitomycin C (see Section 1.4, page 9), where their reactivities are taken advantage of for therapeutic purposes.
The most frequently encountered three-membered heterocycles are oxirane, thiirane, aziridine, and azirine.
Four-membered heterocycles include oxetane, 2H-oxete, thietane, 2H-thiete, azetidine, and azete. In the field of drug discovery, oxetanes and azetidines are more and more incorporated into drugs for modulating biological and physical properties as well as for expanding intellectual properties space.
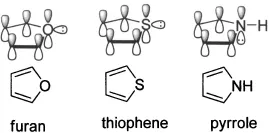
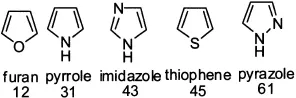
Five- and six-membered heterocycles are of utmost importance to both life and drug discovery. The most common five-membered heterocycles with one heteroatom are pyrrole, furan, and thiophene.
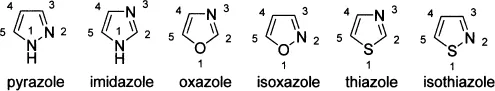
Popular five-membered heterocycles with two heteroatoms include pyrazole, imidazole, oxazole, isoxazole, thiazole, and isothiazole.
All these aromatic heterocycles have their counterparts in the corresponding saturated heterocycles. Among those, pyrrolidines, tetrahydrofurans, and oxazolidines are more frequently encountered in drug discovery.
Some of the important benzene-fused five-membered heterocycles are indole, benzofuran, benzothiophene, benzimidazole, benzoxazole, and benzothiazole. The numbering of these heterocycles is shown below:
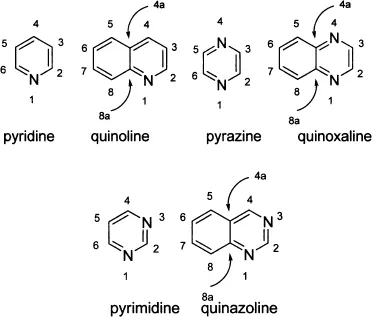
Chief among the six-membered heterocycles, pyridine and its benzene-fused derivative quinoline are most ubiquitous. Pyrazine and its benzene-fused analogue, quinoxaline, also play an important role in heterocyclic chemistry.
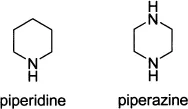
Their corresponding saturated derivatives often encountered in drug discovery are piperidine and piperazine.
1.2 Aromaticity of Heterocycles
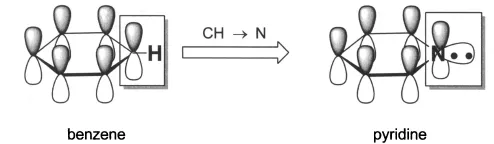
The major thrust of this book is aromatic heterocycles. According to Hückel’s rule of aromaticity, a cyclic ring molecule is aromatic when the number of its π-electrons equals 4n + 2, where n is zero or any positive integer. The most common aromatic compound is benzene, which has 4 + 2 = 6 π-electrons. Pyridine, an electron-deficient aromatic heterocycle, also has 6 π-electrons. In comparison with benzene, pyridine has an additional lone pair of electrons at the nitrogen atom after it contributes a pair of two electrons to make up the 6 π-electrons for aromaticity. These lone pair electrons are responsible for much of pyridine’s unique physical and chemical properties. On the other hand, furan, an electron-excessive aromatic heterocycle also with 6 π-electrons, is different from both benzene and pyridine. The oxygen atom has two lone pairs of electrons, one of which contributes to the 6 π-electrons to achieve the aromaticity. The second pair of electrons is located in an sp2 hybrid orbital in the plane of the furan ring. Thiophene is similar to furan in its aromaticity although thiophene is more “aromatic” because the S atom is larger than the O atom.
The relative aromaticity of common heterocycles is shown below:
Pyrrole, also an aromatic heterocycle with 6 π-electrons, is probably the most unique of all among the aromatic heterocycles. Different from furan and thiophene, the nitrogen atom on the pyrrole ring only has one lone pair of electrons, which both contributed to the 6 π-electrons to achieve the aromaticity. As a consequence, although pyrrole is also an electron-excessive aromatic heterocycle, just like furan and thiophene, pyrrole has many of its own characteristics. For instance, it is probably the most reactive as a nucleophile among all aromatic heterocycles (see Chapter 2). In addition, pyrrole’s conjugation effect outweighs the nitrogen’s inductuve effect in the contributing dipole moment, with the partial positive charge resting at the nitrogen atom.
1.3 Importance of Heterocycles in Life
The importance of heterocycles in life was recognized as the nascent stage of organic chemistry two centuries ago with isolation of alkaloids such as morphine from poppy seeds, quinine from cinchona barks, and camptothecin from the Chinese joy tree. Today, heterocycles are found in numerous fields of biochemical and physiological such as photosynthesis, amino acids, DNA bases, vitamins, endogenous neurotransmitters, and so on.
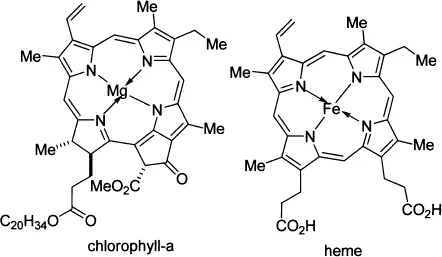
To begin with, chlorophyll is porphyrin (a tetramer of pyrrole) surrounding a magnesium atom. It is the molecule that absorbs sunlight and uses its energy to synthesize carbohydrates from CO2 and water. This process, known as photosynthesis, is the basis for sustaining the life processes of all plants.
On the other hand, the heme consists of a porphyrin ring surrounding an iron atom. The ring contains a large number of conjugated double bonds, which allows the molecule to absorb light in the visible...