This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Book details
Book preview
Table of contents
Citations
About This Book
Reflecting the rapid progress in the field, the book presents the current understanding of molecular mechanisms of post-transcriptional gene regulation thereby focusing on RNA processing mechanisms in eucaryotic cells. With chapters on mechanisms as RNA splicing, RNA interference, MicroRNAs, RNA editing and others, the book also discusses the critical role of RNA processing for the pathogenesis of a wide range of human diseases. The interdisciplinary importance of the topic makes the title a useful resource for a wide reader group in science, clinics as well as pharmaceutical industry.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Post-Transcriptional Gene Regulation by Jane Wu in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Genetics & Genomics. We have over one million books available in our catalogue for you to explore.
Information
1
The Role of Cotranscriptional Recruitment of RNA-Binding Proteins in the Maintenance of Genomic Stability
1.1 Introduction
All steps in transcription and pre-mRNA processing are extensively coordinated. The carboxy-terminal domain (CTD) of the large subunit of RNA polymerase (RNAP) II plays an important role in cotranscriptionally recruiting factors necessary for capping, splicing, polyadenylation, and other mRNA processing events [1–4]. The CTD acts as a platform for these factors to bind, and this process is coordinated by phosphorylation changes that occur during transcription [5]. Although transcription and RNA processing steps are not obligatorily coupled, as seen by the fact that these processes have been studied for many years as individual steps, some posttranscriptional modifications have been shown to be functionally coupled in vitro such as transcription capping [6] and transcription 3′ end processing [7]. There has also been recent evidence for “reverse coupling,” where a proximal 5′ splice site enhances the recruitment of basal transcription initiation factors to the promoter [8]. While it is still unclear if transcription and splicing are functionally coupled in the cell [9, 10], there is evidence that cotranscriptional recruitment of serine-arginine (SR) proteins onto pre-mRNA is vital in maintaining genomic stability [11, 12]. Indeed, recent work shows that ASF/SF2, an SR protein first discovered for its role in constitutive and alternative splicing [13, 14], is a component of a high-molecular-weight (HMW) complex formed on pre-mRNA during cotranscriptional splicing assays [15], reflecting the early recruitment of ASF/SF2 and other SR proteins to nascent RNA during transcription.
All the steps in transcription and RNA processing appear to function together to produce an export-competent and translatable mRNP. In yeast, where splicing is less frequent, transcription is coupled to loading of export factors and mRNP formation through the THO/TREX complex [16]. Mutation of factors in the THO/TREX complex also results in genomic instability [16]. In metazoans, transcription is linked to mRNP formation through splicing [17], formation of the exon junction complex (EJC) [18, 19], and THO/TREX recruitment [16].
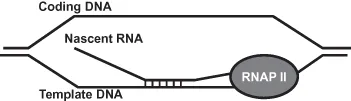
Below we will discuss the coordination of transcription and pre-mRNA processes that inherently protects the genome from invasion of nascent RNA into DNA of the transcribing locus. The invading RNA can then hybridize to the template DNA, producing an aberrant R-loop structure, leaving the coding strand of the DNA single stranded and subject to DNA damage and strand breakage (Figure 1.1). We will describe other examples of cotranscriptionally formed R-loops and speculate on mechanisms that cause such structures to lead to genomic instability.
Figure 1.1 Schematic of a cotranscriptionally formed R-loop structure. Nascent RNA hybridizes with template DNA, leaving coding DNA single stranded.
1.2 THO/TREX
1.2.1 THO/TREX in Saccharomyces cerevisiae
The THO complex proteins were first discovered in genetic screens for their role in transcription elongation of GC-rich genes in S. cerevisiae [20]. The complex consists of Hpr1, Tho2, Mft2, and Thp2, which are recruited to elongating RNAP II complexes. In addition to impairment of transcriptional elongation, THO mutants cause reduced efficiency of gene expression and an increase in hyper-recombination between direct repeats [21]. Mutations in hpr1, tho2, and mft1 can also produce export defects and retention of transcripts at sites of transcription [16]. This reflects the association of THO with export factors Yra1 and Sub1 to form the TREX complex (transcription/export). TREX is recruited early to actively transcribing genes and travels entire length of genes with RNAP II [16]. Interestingly, mutants of the export machinery, Sub2, Yra1, Mex67, and Mtr2 also have THO-like phenotypes of defective transcription and hyper-recombination [16]. Further investigation of 40 selected mutants representing various steps in biogenesis and export of mRNP showed a weak but significant effect on recombination and transcript accumulation [22]. In particular, mutants of the nuclear exosome and 3′ end processing machinery showed inefficient transcription elongation and genetic interactions with THO. The TREX complex exemplifies the importance of the link between transcription and export-competent mRNP formation in yeast with the maintenance of the genomic integrity.
Further investigation of the association of the yeast TREX complex with actively transcribed DNA showed that the THO components play a critical role in the loading of the export machinery onto newly synthesized RNA [23]. Hpr1 was shown to associate with DNA templates through its association with the CTD. While Sub2 was only bound to nascent RNA, Yra1 was associated with both DNA and RNA on intronless genes. Yra1 is recruited to THO and helps to load Sub2 onto the nascent RNA. While Hpr1 was able to associate with both intronless and intron-containing templates similarly, there was a large decrease in the ability of Yra1p and Sub2 to be deposited onto the intron-containing RNA. Data suggested that spliceosome assembly interfered with the transfer of TREX components onto the RNA in these in vitro transcription assays.
1.2.2 THO/TREX in Higher Eukaryotes
Recruitment of TREX may not be transcription coupled in mammals but coupled to splicing instead [24]. When Tho2 immunodepleted HeLa nuclear extracts were used for in vitro transcription, there was no elongation defect detected, as was seen in yeast. There was also no effect on spliceosome assembly, splicing, or RNA stability even though all components of the THO complex have previously been detected in purified spliceosomes [25]. Immunoprecipitation assays showed that human Tho2 only associated with in vitro spliced mRNA but not unspliced pre-mRNA. Further in vitro experiments showed that TREX bound to the 5′ cap-binding complex (CBC) in a splicing-dependent manner [26]. Immunoprecipitation assays showed that TREX preferentially associated with in vitro spliced and capped mRNA compared with uncapped or unspliced. The interaction of TREX with the 5′ cap is mediated by protein–protein interactions between REF/Aly and CBP80. Microinjection of these preassembled mRNP into Xenopus oocytes showed then to be export competent.
The above in vitro data seem to conflict with the in vivo data produced by Hrp1 depletion in HeLa cells. While it is evident that export-component REF/Aly directly interacts with CBP80 in a splicing-dependent manner, Hpr1 associates with DNA not RNA in yeast [23], so it is possible that recruitment of hHpr1 and hTho2 to the CBC in the immunoprecipitation assay may be due to their affinity for REF/Aly. Also, in Drosophila melanogaster, only the depletion of both THO2 and HPR1 by siRNA shows significant nuclear accumulation of poly(A)+ RNA [27], which could signify their divergent roles in transcription elongation and formation of export-component mRNP. THO is essential for heat-shock mRNA export in D. melanogaster, which may perhaps reflect its role in stress conditions. If the recruitment of REF/Aly is only dependent on cap formation and splicing, this might also explain the THO-independent recruitment of UAP56 in D. melanogaster. In any event, these data together indicate that the recruitment of the export machinery in higher eukaryotes is not linked to THO/TREX in a manner similar to S. cerevisiae.
1.2.3 THO/TREX and R-loop Formation
How do defects in THO/TREX cause hyper-recombination? Huertas and Aguilera proposed that mutations affecting THO/TREX components cause cotranscriptional production of aberrant R-loop structures [28]. They provided evidence of R-loop formation utilizing a hammerhead ribozyme to release hybridized nascent RNA. This ribozyme was able to suppress transcription elongation impairment and hyper-recombination phenotype in THO mutants. Further evidence was provided by overexpression of RNase H to degrade RNA moiety of RNA:DNA hybrids, which also suppressed the THO phenotypes [28]. A more recent study of the point mutant hpr1-101, which has a transcriptional defect but does not cause R-loop formation, shows no hyper-recombination phenotype. This indicates that while the transcriptional defect by THO mutants may be further aggravated by R-loops, they are not mediated by them. The RNA:DNA hybrids do appear to lead to the hyper-recombination phenotype associated with THO mutants [29].
Therefore, in yeast, early recruitment of THO/TREX plays a key role in protecting against or preventing the formation of R-loop structures. In mammals though, the late recruitment of THO/TREX suggests that it plays a less important role, or perhaps no role, in protecting against genomic instability. This points to a possible role for earlier cotranscriptional processes in protecting the genome from DNA damage.
1.3 Linking Transcription to Export of mRNP
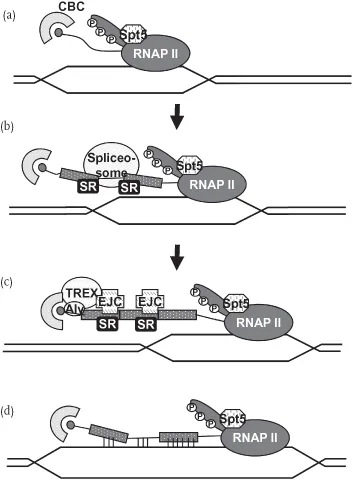
Early in transcription, the cell is already preparing to package nascent pre-mRNA into mRNP. As mentioned earlier, the RNAP II CTD coordinates the recruitment of RNA processing factors to transcribing genes. Spt5, a subunit of the DRB sensitivity-inducing factor (DSIF) transcriptional elongation factor, plays an early role in integrating the various steps of pre-mRNA processing to guarantee an export-competent mRNP. Immediately after transcription is induced, Spt5 helps to recruit a capping enzyme (CE) [30, 31], which is then activated together with the phosphorylated CTD to cap the 5′ end of the growing pre-mRNA (Figure 1.2a). In vitro, the recruitment of CE has been shown to cause the formation of R-loops [32]. However, these may be prevented by the recruitment of ASF/SF2 to the RNA, which prevents the formation of these aberrant RNA:DNA structures [11, 32]. Spt5 has been implicated not only in transcription elongation [33], CE recruitment, and splicing [34, 35], but also in transcription-coupled repair [36] and the recruitment of the exosome [37], which plays a key role in mRNP quality control [38, 39].
Figure 1.2 Early steps in cotranscriptional mRNA processing protect against genomic instability. (a) The phosphorylated CTD of RNAP II, along with Spt5, recruits a capping enzyme to cap the 5′ end of a growing pre-mRNA. These steps work to (b) cotranscriptionally load the spliceosome and SR proteins onto pre-mRNA to remove the introns. (c) The EJC is then deposited onto the mRNA in a splicing-dependent manner. REF/Aly and other components of the TREX complex are stabilized onto the 5′ end of the mRNA. (d) Disruption of any of these early steps of mRNA processing can leave nascent RNA open to bind to template DNA forming an R-loop structure.
1.3.1 The Thp1-Sac3-Sus1-Cdc31 (THSC) Complex
In S. cerevisiae, in addition to THO/TREX, another complex has been shown to play a role in the export of properly formed mRNPs. The Thp1-Sac3-Sus1-Cdc31 (THSC) compl...
Table of contents
- Cover
- Related Titles
- Title page
- Copyright page
- Foreword
- List of Contributors
- 1 The Role of Cotranscriptional Recruitment of RNA-Binding Proteins in the Maintenance of Genomic Stability
- 2 Transcription Termination by RNA Polymerase II
- 3 Posttranscriptional Gene Regulation by an Editor: ADAR and its Role in RNA Editing
- 4 Posttranslational Modification of Sm Proteins: Diverse Roles in snRNP Assembly and Germ Line Specification
- 5 Structure, Function, and Biogenesis of Small Nucleolar Ribonucleoprotein Particles
- 6 Mechanistic Insights into Mammalian Pre-mRNA Splicing
- 7 Splicing Decisions Shape Neuronal Protein Function across the Transcriptome
- 8 Noncoding RNA: The Major Output of Gene Expression
- 9 Noncoding RNAs, Neurodevelopment, and Neurodegeneration
- 10 The Evolution of the Modern RNA World
- Index