
eBook - ePub
Polyurethanes
Science, Technology, Markets, and Trends
Mark F. Sonnenschein
This is a test
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
Polyurethanes
Science, Technology, Markets, and Trends
Mark F. Sonnenschein
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
This book, cohesively written by an expert author with supreme breadth and depth of perspective on polyurethanes, provides a comprehensive overview of all aspects of the science and technology on one of the most commonly produced plastics.
- Covers the applications, manufacture, and markets for polyurethanes, and discusses analytical methods, reaction mechanisms, morphology, and synthetic routes
- Provides an up-to-date view of the current markets and trend analysis based on patent activity and updates chapters to include new research
- Includes two new chapters on PU recycling and PU hybrids, covering the opportunities and challenges in both
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist Polyurethanes als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu Polyurethanes von Mark F. Sonnenschein im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Technologie et ingénierie & Ingénierie de la chimie et de la biochimie. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
1
INTRODUCTION
In the early 1900s there were very few of the synthetic polymers we have grown accustomed to now. During succeeding years polymer science experienced explosive growth with the invention of polyvinyl chloride (PVC, 1913), polyethylene (1933), polyvinylidene chloride (Saran, 1933), polyamides (nylon, 1934), and polytetrafluoroethylene (Teflon, 1938). In addition, during the 1930s the polymer family known as polyurethanes was invented. Now, of course, polyurethanes, and all the polymers developed during this period, have become an integral part of modern life. As you read this you may not be aware of how many ways polyurethanes surround you. They are present in the shoes you stand in, the seat cushion you sit upon, the carpet backing and foam pad underlay you walk upon, in the fibers of your clothing, insulation of your walls and roof, in your refrigerator, dishwasher, water heater, automotive seating, automotive structural foam, automotive paints and coatings, furniture coatings, your bed mattress, the adhesive holding this book together – the list just goes on. This book’s purpose is to explain polyurethane science, technology, applications, trends, and markets in virtually all of its forms and relate those structures to the properties that make them so suited for so many uses. It is not an overstatement to say that if polyurethanes are not the most versatile class of materials, then they are certainly one of the most versatile polymer categories in existence.
Discovery of polyurethane chemistry is attributed to the efforts of Otto Bayer and the research team he led at the now defunct I.G. Farben AG chemical company. The first patent associated with polyurethanes was filed in 1937 and numerous other patents, most notably the production of flexible foams resulting from isocyanate–water reactions, were filed thereafter. I.G. Farben was broken up following World War II for complicity in war crimes and the company’s top leaders were convicted of crimes against humanity (exploitation of slave labor and production of nerve gas). The largest surviving components of I.G. Farben – Bayer AG and BASF SE – remain very large and respected global industrial concerns. While BASF continues to engage with and maintain a significant presence within the polyurethane industry, Bayer spun off its polyurethane business and the rest of its industrial chemicals concerns into a new company called Covestro.
After the initial discovery and expositions of basic chemistry, mostly based on short‐chain diols and polyester polyols, industrial polyurethanes saw immense growth following the development of polyether polyols by E.I. du Pont de Nemours and Company (now known as DuPont) and The Dow Chemical Company. While Dow Chemical remains one of the world’s largest manufacturers of polyurethane chemicals, DuPont has exited its polyurethanes businesses, which were primarily textile and coatings related. While polyesters remain prominent components of polyurethane chemistry, it was the superior processing, low‐temperature flexibility, and hydrolytic stability of polyether polyols that expanded polyurethane polymers into their current acceptance in every aspect of modern life.
As ubiquitous as polyurethanes are, it is perhaps surprising that they represent a relatively minor (but still significant) fraction of the overall global consumption of plastics by volume (Figure 1.1).
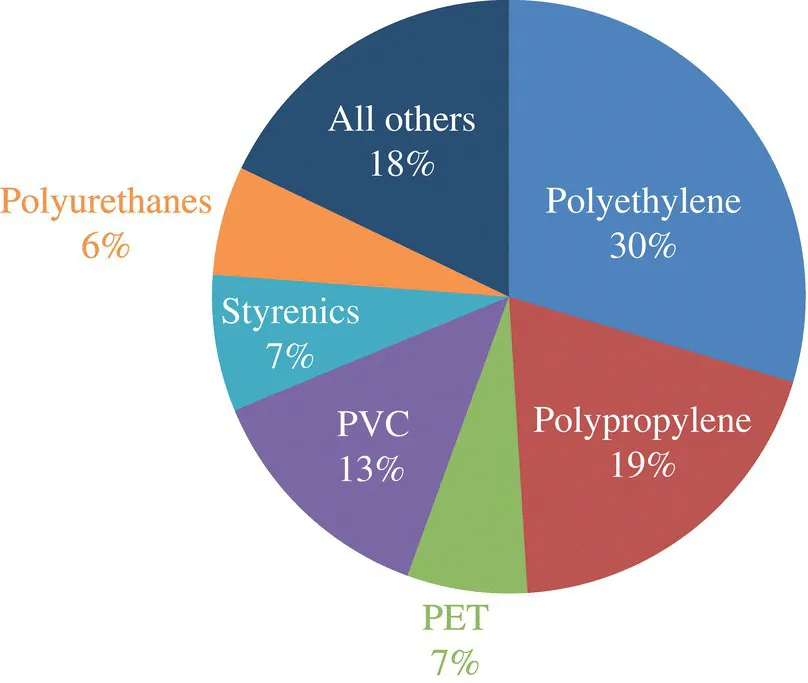
FIGURE 1.1 Percentage global consumption of plastics in 2018. Polyethylene encompasses all densities; styrenics includes all copolymers along with atactic polystyrene. These relative values are similar to those in the first edition using 2012 data. The consumption of many plastics grows at a rate relative to economic activity plus a small accelerator or decelerator for each given plastic’s role in the market. PET = polyethyene terephthate.
The structures of the listed commodity polymers are relatively simple repeating units (Figure 1.2). Their simplicity is in part responsible for their high level of utility and low‐cost positions. The plastics industry has generated variants of the structures shown in Figure 1.2 by, for instance, introducing branches, but these complexities do not fundamentally alter the basic polymer structure.
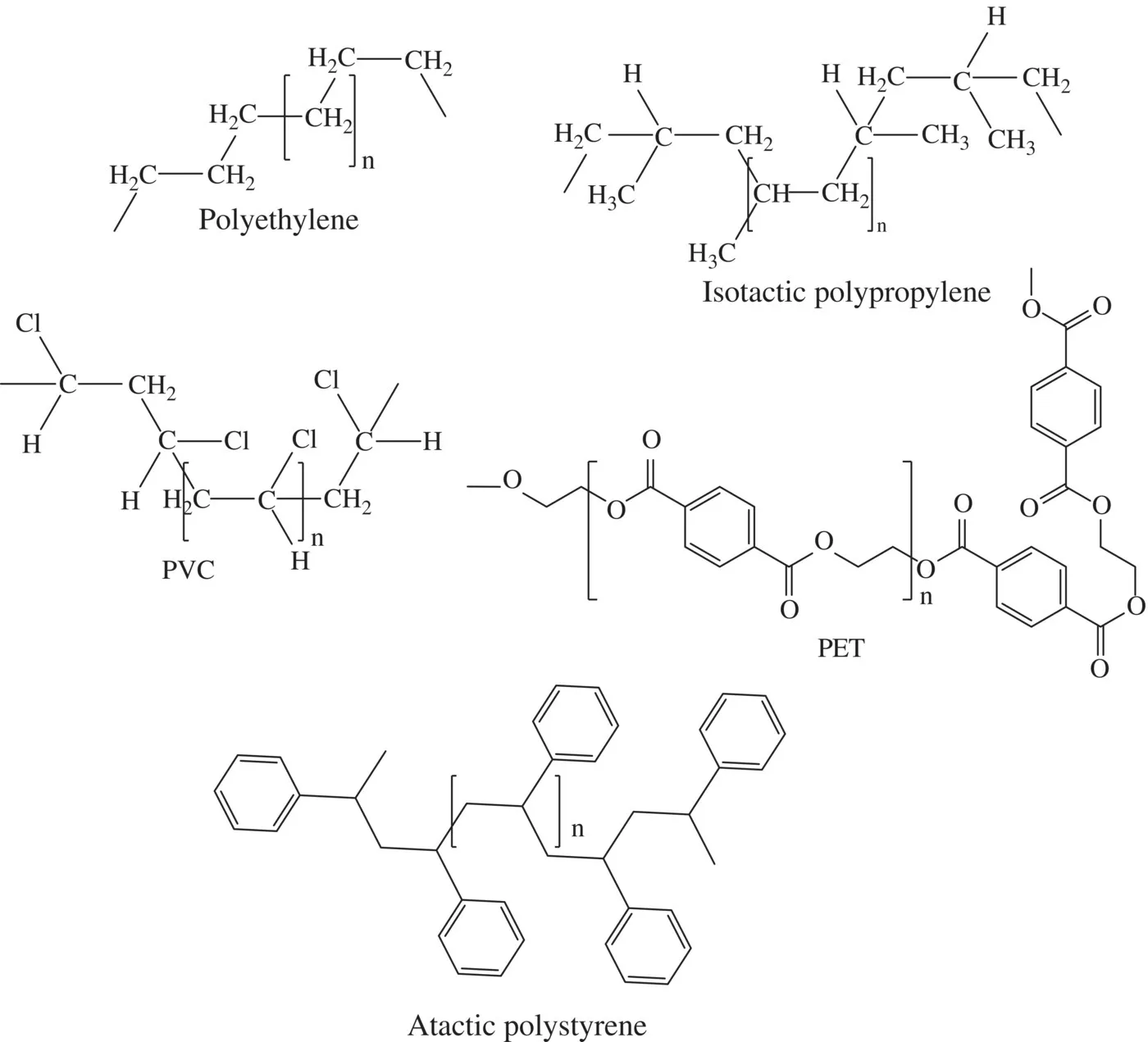
FIGURE 1.2 Illustrative structures of high‐volume commodity polymers.
Polyurethane is the largest volume commodity polymer that cannot be characterized by a simple structure such as that shown in Figure 1.2. Instead, polyurethane represents a class of polymers, and any polymer with a urethane repeat unit is classified as a polyurethane regardless of the other functional or polymer structures incorporated (Figure 1.3).
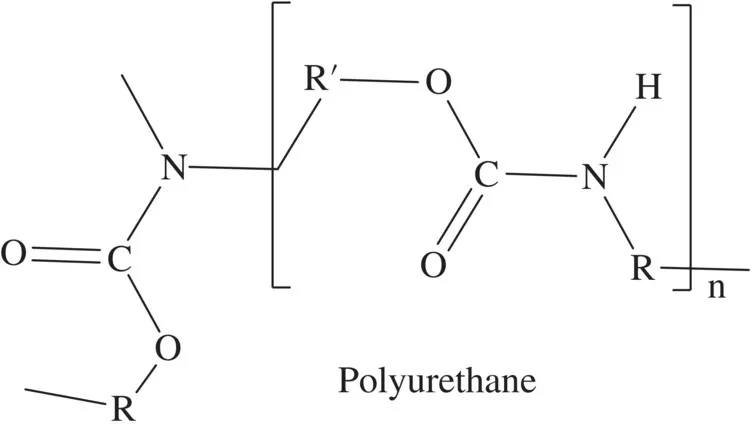
FIGURE 1.3 The urethane unit within a polyurethane polymer chain.
Specific polyurethane structures used for making mattress foam, insulation foam, or shoe foam can be significantly different from one another and cannot be neatly represented like the structures in Figure 1.2. In fact, even structures of different insulation foams can vary so widely that they also cannot be easily represented by a single structure. Another difference with other commodity polymers is that large‐volume polyurethane applications require the mixing of two reactive liquid components rather than the processing of a pellet into a molded or extruded object. Given these complexities it is remarkable that polyurethanes have developed into a commodity plastic category, and it is testament to the versatility and performance of polyurethanes that they are so difficult to replace in their favored applications.
Polyurethane polymers as a class are made from commodity building block reagents and short‐chain polymers (or oligomers). These building blocks include, for example, the following categories: polyisocyanates, polyethers, polyesters, water, and amines (Figure 1.4). As building block categories they also cannot be represented by unique structures and are denoted by “R” to allow designers to insert any conceivable chemically allowable unit.
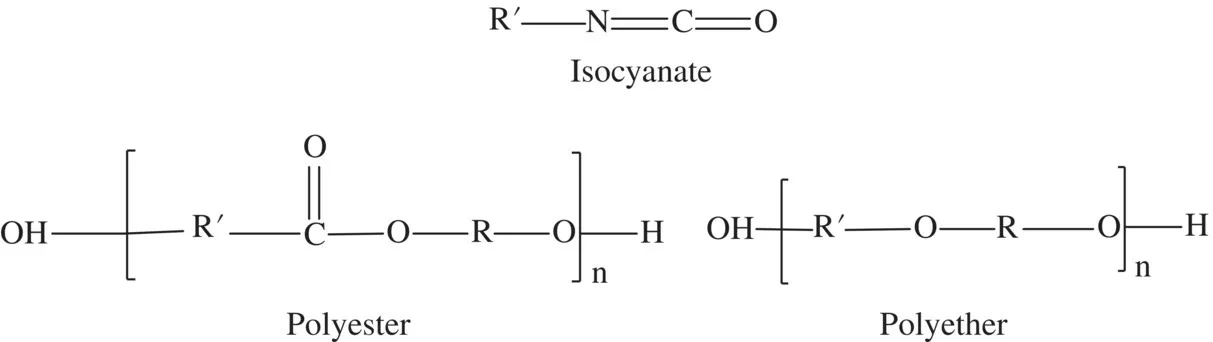
FIGURE 1.4 Chemical structures of isocyanate, polyester, and polyether. To make a polyurethane the Rʹ of the isocyanate structure must also have an isocyanate function [1].
The polyurethane unit is easily mistaken for the related polyester, polyurea, or polyamide (nylon) structures (Figure 1.5). In fact, polyureas, polyesters, and polyurethanes are often joined into polyurethane materials and still broadly classified as polyurethane. (Polyamides were not previously a part of polyurethane chemistry because of their vastly different processing characteristics. However, recent literature indicates nascent explorations of urethane–amide hybrids; see Chapter 13.)
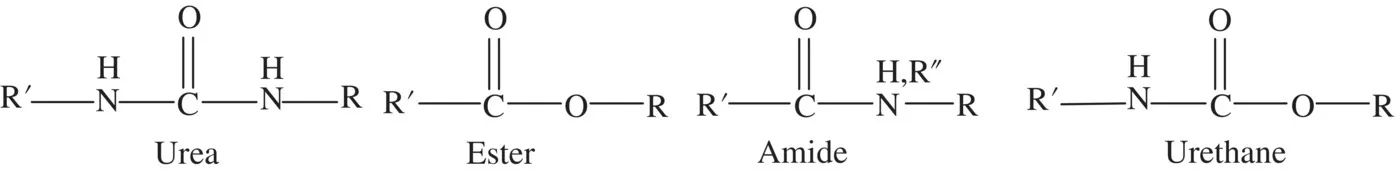
FIGURE 1.5 Structures of urea, ester, amide, and urethane functionalities.
As commodity products, polyurethanes have achieved a certain establishment status in academic ...
Inhaltsverzeichnis
- COVER
- TABLE OF CONTENTS
- TITLE PAGE
- COPYRIGHT PAGE
- DEDICATION PAGE
- PREFACE
- ACKNOWLEDGMENTS
- 1 INTRODUCTION
- 2 POLYURETHANE BUILDING BLOCKS
- 3 INTRODUCTION TO POLYURETHANE CHEMISTRY
- 4 THEORETICAL CONCEPTS AND TECHNIQUES IN POLYURETHANE SCIENCE
- 5 ANALYTICAL CHARACTERIZATION OF POLYURETHANES
- 6 POLYURETHANE FLEXIBLE FOAMS
- 7 POLYURETHANE FLEXIBLE FOAMS
- 8 POLYURETHANE RIGID FOAMS
- 9 POLYURETHANE ELASTOMERS
- 10 POLYURETHANE ADHESIVES AND COATINGS
- 11 SPECIAL TOPIC
- 12 SPECIAL TOPIC
- 13 POLYURETHANE HYBRID POLYMERS
- 14 RECYCLING OF POLYURETHANES
- INDEX
- END USER LICENSE AGREEMENT
Zitierstile für Polyurethanes
APA 6 Citation
Sonnenschein, M. (2020). Polyurethanes (2nd ed.). Wiley. Retrieved from https://www.perlego.com/book/2051390/polyurethanes-science-technology-markets-and-trends-pdf (Original work published 2020)
Chicago Citation
Sonnenschein, Mark. (2020) 2020. Polyurethanes. 2nd ed. Wiley. https://www.perlego.com/book/2051390/polyurethanes-science-technology-markets-and-trends-pdf.
Harvard Citation
Sonnenschein, M. (2020) Polyurethanes. 2nd edn. Wiley. Available at: https://www.perlego.com/book/2051390/polyurethanes-science-technology-markets-and-trends-pdf (Accessed: 15 October 2022).
MLA 7 Citation
Sonnenschein, Mark. Polyurethanes. 2nd ed. Wiley, 2020. Web. 15 Oct. 2022.