
eBook - ePub
Transition Metal-Catalyzed Pyridine Synthesis
Transition Metal-Catalyzed Heterocycle Synthesis Series
Xiao-Feng Wu
This is a test
- 90 páginas
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
eBook - ePub
Transition Metal-Catalyzed Pyridine Synthesis
Transition Metal-Catalyzed Heterocycle Synthesis Series
Xiao-Feng Wu
Detalles del libro
Vista previa del libro
Índice
Citas
Información del libro
Transition Metal-Catalyzed Pyridine Synthesis provides an overview of pyridines, describing properties of these heterocycle compounds and describing traditional synthetic procedures for them. The book then explores catalyzed procedures for pyridine synthesis in greater detail and depth than is currently available in published Review articles.
The short series Transition Metal-Catalyzed Heterocycles Synthesis, authored by Xiao-Feng Wu, summarizes recent achievements on heterocycles synthesis with transition metal as the catalysts, with each volume dedicated to one heterocycle compound.
- Brief, focused review of this active research area, Pyridine synthesis via transition metal catalysis
- Useful coverage of Pyridine properties and both intermolecular and intramolecular cyclization
- Volume Two in Elsevier's short work series, "Transition Metal-Catalyzed Heterocycles Synthesis"
Preguntas frecuentes
¿Cómo cancelo mi suscripción?
¿Cómo descargo los libros?
Por el momento, todos nuestros libros ePub adaptables a dispositivos móviles se pueden descargar a través de la aplicación. La mayor parte de nuestros PDF también se puede descargar y ya estamos trabajando para que el resto también sea descargable. Obtén más información aquí.
¿En qué se diferencian los planes de precios?
Ambos planes te permiten acceder por completo a la biblioteca y a todas las funciones de Perlego. Las únicas diferencias son el precio y el período de suscripción: con el plan anual ahorrarás en torno a un 30 % en comparación con 12 meses de un plan mensual.
¿Qué es Perlego?
Somos un servicio de suscripción de libros de texto en línea que te permite acceder a toda una biblioteca en línea por menos de lo que cuesta un libro al mes. Con más de un millón de libros sobre más de 1000 categorías, ¡tenemos todo lo que necesitas! Obtén más información aquí.
¿Perlego ofrece la función de texto a voz?
Busca el símbolo de lectura en voz alta en tu próximo libro para ver si puedes escucharlo. La herramienta de lectura en voz alta lee el texto en voz alta por ti, resaltando el texto a medida que se lee. Puedes pausarla, acelerarla y ralentizarla. Obtén más información aquí.
¿Es Transition Metal-Catalyzed Pyridine Synthesis un PDF/ePUB en línea?
Sí, puedes acceder a Transition Metal-Catalyzed Pyridine Synthesis de Xiao-Feng Wu en formato PDF o ePUB, así como a otros libros populares de Physical Sciences y Organic Chemistry. Tenemos más de un millón de libros disponibles en nuestro catálogo para que explores.
Información
Categoría
Physical SciencesCategoría
Organic ChemistryChapter 1
Introduction
Abstract
A short introduction on pyridine derivative has been given.
Keywords
Pyridine; biological active; organic synthesis
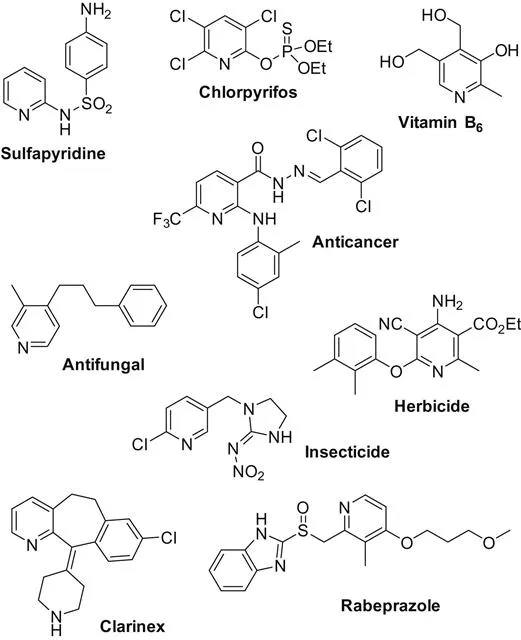
Pyridine is an important class of nitrogen-containing heterocycles found in various natural products, pharmaceuticals, and materials (Scheme 1.1). Based on its importance, numerous synthetic procedures have been developed for their preparation [1]. In this book volume, the main achievements on transition metal-catalyzed pyridines synthesis are discussed. Based on the reaction types, the whole volume is catalogued by intramolecular cyclization and intermolecular cyclization reactions.
Chapter 2
Synthesized by Intramolecular Cyclizations
Abstract
The procedures based on transition metal-catalyzed intramolecular cyclizations to prepare pyridines have been discussed here.
Keywords
Pyridine; intramolecular cyclization; organic synthesis; synthetic methodology; carbonylation; coupling
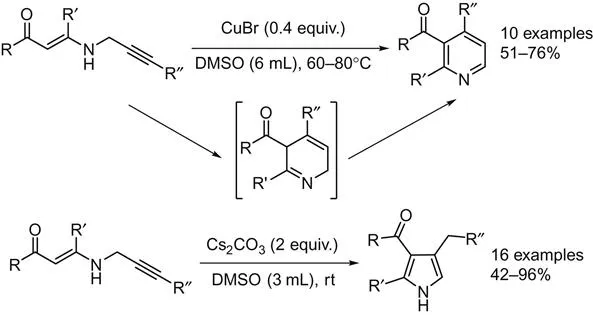
Intramolecular cyclization of substrates to the corresponding pyridine derivatives is the most straightforward pathway. By intramolecular version reaction, the positions of substituents are fixed and easier to control, such as N-propargylic β-enaminones in pyridines and pyrroles synthesis. In 2008, Cacchi et al. reported a copper-catalyzed synthesis of polysubstituted pyridines from N-propargylic β-enaminones [1]. By using DMSO as the solvent, pyrroles can be produced in good to high yields in the presence of only Cs2CO3 at room temperature, while pyridines can be formed under the assistant of CuBr at 60–80°C (Scheme 2.1). Here, the N-propargylic β-enaminones can be prepared via the following sequences: (1) cross-coupling of terminal alkynes with acyl chlorides; (2) followed by the conjugate addition of propargylamine with the resultant α,β-enones; (3) further Sonogashira cross-coupling of the propargyl derivative with aryl halides.
A procedure by using enamino ester and alkynone as the substrates was developed as well [2]. 2,3,4,6-Tetrasubstituted pyridines were prepared in a single step. Various acids, such as acetic acid, Amberlyst 15 ion exchange resin, zinc(II) bromide or ytterbium(III) triflate, can be applied as promoter for the cyclization step of the Michael addition adduct. 4-(3-Oxoalkyl)isoxazoles found could be applied as starting material for pyridine synthesis as well [3].
In 2013, Kim and coworkers reported a palladium-catalyzed domino cyclization of N-(2-bromoallyl)-N-cinnamyltosylamides for the construction of pyridines [4]. The reaction proceeds via a domino 5-exo/3-exo carbopalladation, ring expansion by palladium rearrangement, and an aromatization. Various 4-arylnicotinate derivatives were produced in good yields (Scheme 2.2).
Índice
- Cover image
- Title page
- Table of Contents
- Copyright
- Dedication
- Chapter 1. Introduction
- Chapter 2. Synthesized by Intramolecular Cyclizations
- Chapter 3. Synthesized by Intermolecular Cyclizations
- Chapter 4. Summary and Outlook
Estilos de citas para Transition Metal-Catalyzed Pyridine Synthesis
APA 6 Citation
Xiao-Feng. (2016). Transition Metal-Catalyzed Pyridine Synthesis ([edition unavailable]). Elsevier Science. Retrieved from https://www.perlego.com/book/1830185/transition-metalcatalyzed-pyridine-synthesis-transition-metalcatalyzed-heterocycle-synthesis-series-pdf (Original work published 2016)
Chicago Citation
Xiao-Feng. (2016) 2016. Transition Metal-Catalyzed Pyridine Synthesis. [Edition unavailable]. Elsevier Science. https://www.perlego.com/book/1830185/transition-metalcatalyzed-pyridine-synthesis-transition-metalcatalyzed-heterocycle-synthesis-series-pdf.
Harvard Citation
Xiao-Feng (2016) Transition Metal-Catalyzed Pyridine Synthesis. [edition unavailable]. Elsevier Science. Available at: https://www.perlego.com/book/1830185/transition-metalcatalyzed-pyridine-synthesis-transition-metalcatalyzed-heterocycle-synthesis-series-pdf (Accessed: 15 October 2022).
MLA 7 Citation
Xiao-Feng. Transition Metal-Catalyzed Pyridine Synthesis. [edition unavailable]. Elsevier Science, 2016. Web. 15 Oct. 2022.