
eBook - ePub
Transition Metal-Catalyzed Pyridine Synthesis
Transition Metal-Catalyzed Heterocycle Synthesis Series
Xiao-Feng Wu
This is a test
- 90 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Transition Metal-Catalyzed Pyridine Synthesis
Transition Metal-Catalyzed Heterocycle Synthesis Series
Xiao-Feng Wu
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
Transition Metal-Catalyzed Pyridine Synthesis provides an overview of pyridines, describing properties of these heterocycle compounds and describing traditional synthetic procedures for them. The book then explores catalyzed procedures for pyridine synthesis in greater detail and depth than is currently available in published Review articles.
The short series Transition Metal-Catalyzed Heterocycles Synthesis, authored by Xiao-Feng Wu, summarizes recent achievements on heterocycles synthesis with transition metal as the catalysts, with each volume dedicated to one heterocycle compound.
- Brief, focused review of this active research area, Pyridine synthesis via transition metal catalysis
- Useful coverage of Pyridine properties and both intermolecular and intramolecular cyclization
- Volume Two in Elsevier's short work series, "Transition Metal-Catalyzed Heterocycles Synthesis"
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Transition Metal-Catalyzed Pyridine Synthesis est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Transition Metal-Catalyzed Pyridine Synthesis par Xiao-Feng Wu en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Physical Sciences et Organic Chemistry. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
Sujet
Physical SciencesSous-sujet
Organic ChemistryChapter 1
Introduction
Abstract
A short introduction on pyridine derivative has been given.
Keywords
Pyridine; biological active; organic synthesis
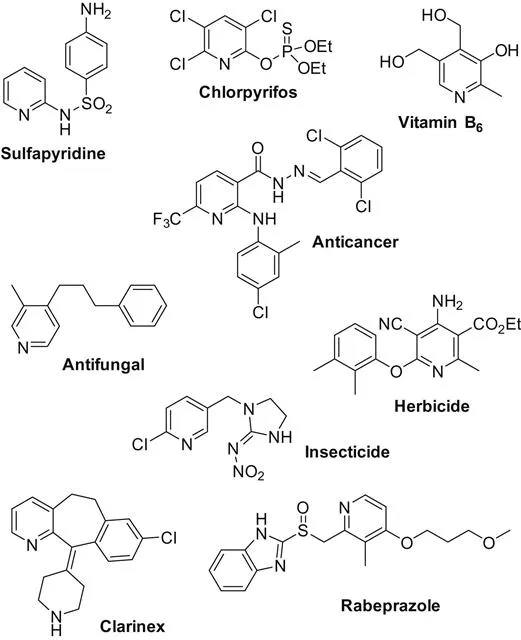
Pyridine is an important class of nitrogen-containing heterocycles found in various natural products, pharmaceuticals, and materials (Scheme 1.1). Based on its importance, numerous synthetic procedures have been developed for their preparation [1]. In this book volume, the main achievements on transition metal-catalyzed pyridines synthesis are discussed. Based on the reaction types, the whole volume is catalogued by intramolecular cyclization and intermolecular cyclization reactions.
Chapter 2
Synthesized by Intramolecular Cyclizations
Abstract
The procedures based on transition metal-catalyzed intramolecular cyclizations to prepare pyridines have been discussed here.
Keywords
Pyridine; intramolecular cyclization; organic synthesis; synthetic methodology; carbonylation; coupling
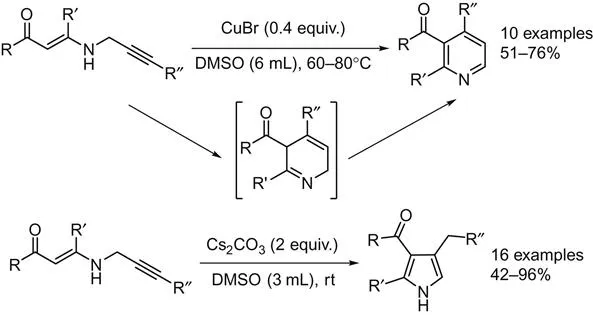
Intramolecular cyclization of substrates to the corresponding pyridine derivatives is the most straightforward pathway. By intramolecular version reaction, the positions of substituents are fixed and easier to control, such as N-propargylic β-enaminones in pyridines and pyrroles synthesis. In 2008, Cacchi et al. reported a copper-catalyzed synthesis of polysubstituted pyridines from N-propargylic β-enaminones [1]. By using DMSO as the solvent, pyrroles can be produced in good to high yields in the presence of only Cs2CO3 at room temperature, while pyridines can be formed under the assistant of CuBr at 60–80°C (Scheme 2.1). Here, the N-propargylic β-enaminones can be prepared via the following sequences: (1) cross-coupling of terminal alkynes with acyl chlorides; (2) followed by the conjugate addition of propargylamine with the resultant α,β-enones; (3) further Sonogashira cross-coupling of the propargyl derivative with aryl halides.
A procedure by using enamino ester and alkynone as the substrates was developed as well [2]. 2,3,4,6-Tetrasubstituted pyridines were prepared in a single step. Various acids, such as acetic acid, Amberlyst 15 ion exchange resin, zinc(II) bromide or ytterbium(III) triflate, can be applied as promoter for the cyclization step of the Michael addition adduct. 4-(3-Oxoalkyl)isoxazoles found could be applied as starting material for pyridine synthesis as well [3].
In 2013, Kim and coworkers reported a palladium-catalyzed domino cyclization of N-(2-bromoallyl)-N-cinnamyltosylamides for the construction of pyridines [4]. The reaction proceeds via a domino 5-exo/3-exo carbopalladation, ring expansion by palladium rearrangement, and an aromatization. Various 4-arylnicotinate derivatives were produced in good yields (Scheme 2.2).
Table des matières
- Cover image
- Title page
- Table of Contents
- Copyright
- Dedication
- Chapter 1. Introduction
- Chapter 2. Synthesized by Intramolecular Cyclizations
- Chapter 3. Synthesized by Intermolecular Cyclizations
- Chapter 4. Summary and Outlook
Normes de citation pour Transition Metal-Catalyzed Pyridine Synthesis
APA 6 Citation
Xiao-Feng. (2016). Transition Metal-Catalyzed Pyridine Synthesis ([edition unavailable]). Elsevier Science. Retrieved from https://www.perlego.com/book/1830185/transition-metalcatalyzed-pyridine-synthesis-transition-metalcatalyzed-heterocycle-synthesis-series-pdf (Original work published 2016)
Chicago Citation
Xiao-Feng. (2016) 2016. Transition Metal-Catalyzed Pyridine Synthesis. [Edition unavailable]. Elsevier Science. https://www.perlego.com/book/1830185/transition-metalcatalyzed-pyridine-synthesis-transition-metalcatalyzed-heterocycle-synthesis-series-pdf.
Harvard Citation
Xiao-Feng (2016) Transition Metal-Catalyzed Pyridine Synthesis. [edition unavailable]. Elsevier Science. Available at: https://www.perlego.com/book/1830185/transition-metalcatalyzed-pyridine-synthesis-transition-metalcatalyzed-heterocycle-synthesis-series-pdf (Accessed: 15 October 2022).
MLA 7 Citation
Xiao-Feng. Transition Metal-Catalyzed Pyridine Synthesis. [edition unavailable]. Elsevier Science, 2016. Web. 15 Oct. 2022.