Biological Sciences
Glycolysis
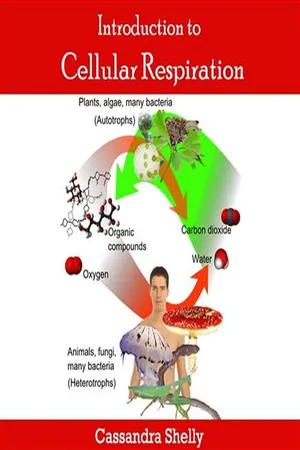
Glycolysis is the metabolic pathway that breaks down glucose into pyruvate, generating ATP and NADH in the process. It occurs in the cytoplasm and is the first step in both aerobic and anaerobic respiration. Glycolysis consists of 10 enzymatic reactions, ultimately producing two molecules of pyruvate, two molecules of ATP, and two molecules of NADH from one molecule of glucose.
Written by Perlego with AI-assistance
Related key terms
1 of 5
10 Key excerpts on "Glycolysis"
- No longer available |Learn more
- (Author)
- 2014(Publication Date)
- The English Press(Publisher)
________________________ WORLD TECHNOLOGIES ________________________ Chapter- 1 Glycolysis Glycolysis overview Glycolysis (from glycose , an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C 6 H 12 O 6 , into pyruvate, CH 3 COCOO − + H + . The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide). Glycolysis is a definite sequence of ten reactions involving ten intermediate compounds (one of the steps involves two intermediates). The intermediates provide entry points to Glycolysis. For example, most monosaccharides, such as fructose, glucose, and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat. ________________________ WORLD TECHNOLOGIES ________________________ Glycolysis is thought to be the archetype of a universal metabolic pathway. It occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of Glycolysis indicates that it is one of the most ancient known metabolic pathways. The most common type of Glycolysis is the Embden-Meyerhof-Parnas pathway (EMP pathway) , which was first discovered by Gustav Embden, Otto Meyerhof and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden-Meyerhof pathway. Overview The overall reaction of Glycolysis is: D -[Glucose] [Pyruvate] + 2 [NAD] + + 2 [ADP] + 2 [P] i 2 + 2 [NADH] + 2 H + + 2 [ATP] The use of symbols in this equation makes it appear unbalanced with respect to oxygen atoms, hydrogen atoms, and charges. - eBook - PDF
- Charlotte W. Pratt, Kathleen Cornely(Authors)
- 2021(Publication Date)
- Wiley(Publisher)
In the cell, glucose combustion requires many steps so that the cell can recover its energy in smaller, more useful quantities. Glycolysis, representing the first ten steps of this process, appears to be an ancient metabolic pathway. The fact that it does not require molecular oxygen suggests that it evolved before photosynthesis increased the level of atmo- spheric O 2 . Overall, Glycolysis is a series of enzyme-catalyzed steps in which a six-carbon glucose molecule is broken down into two three-carbon pyruvate molecules. This catabolic pathway is accompanied by the phosphorylation of two molecules of ADP (to produce 2 ATP) and the reduction of two molecules of NAD + . The net equation for the pathway (ignoring water and protons) is glucose + 2 NAD + + 2 ADP + 2 P i → 2 pyruvate + 2 NADH + 2 ATP It is convenient to divide the 10 reactions of Glycolysis into two phases. In the first (Reactions 1–5), the hexose is phosphorylated and cleaved in half. In the second (Reactions 6–10), the three-carbon molecules are converted to pyruvate (Fig. 13.2). As you examine each of the reactions of Glycolysis described in the following pages, note how the reaction substrates are converted to products by the action of an enzyme (and note how the enzyme’s name often reveals its purpose). Pay attention also to the free energy change of each reaction. Energy is invested at the start of Glycolysis The early reactions of Glycolysis can be considered as preparation for the later, energy- producing reactions. In fact, two of the early reactions require the investment of energy in the form of ATP. - eBook - ePub
Medical Biochemistry
Human Metabolism in Health and Disease
- Miriam D. Rosenthal, Robert H. Glew(Authors)
- 2011(Publication Date)
- Wiley(Publisher)
CHAPTER 4Glycolysis4.1 FUNCTIONS OF Glycolysis
Glycolysis is a metabolic pathway that cleaves glucose into two molecules of pyruvate or lactate. During Glycolysis, some of the energy in the glucose molecule is converted into ATP. Although Glycolysis to pyruvate is an oxidative process, it is not dependent on molecular oxygen. By contrast, the subsequent fate of pyruvate depends on the presence of both mitochondria and sufficient oxygen. In the presence of oxygen, the product of Glycolysis is pyruvate, which is further oxidized by the pyruvate dehydrogenase enzyme complex and the dehydrogenases of the tricarboxylic acid (TCA) cycle. In the absence of sufficient oxygen, or in red blood cells, where mitochondria are absent, lactate is the final product of Glycolysis and there is no net oxidation. Glycolysis is not only the major pathway for oxidizing glucose, but also the main pathway for metabolizing other dietary sugars, such as galactose and fructose.Once glucose has been trapped inside a cell in the form of glucose 6-phosphate, there may be as many as three metabolic options available for the glucose moiety (Fig. 4-1 ). In a hepatocyte, for example, glucose can be oxidized via Glycolysis for the primary purpose of ATP production, stored as glycogen, or oxidized in the pentose phosphate pathway to generate NADPH and ribose for nucleic acid synthesis. Red blood cells, on the other hand, cannot synthesize glycogen; they can, however, metabolize glucose through the pentose phosphate pathway or through Glycolysis.Three possible metabolic fates of glucose: Glycolysis, the pentose phosphate pathway, and glycogen synthesis.FIGURE 4-14.1.1 Glycolysis Provides Energy
The main function of Glycolysis is energy (ATP) production. The conversion of one molecule of glucose to pyruvate or lactate is associated directly with the net production of two ATP molecules. Since the maximum number of ATP molecules that can be realized from the complete oxidation of one molecule of glucose to CO2 - No longer available |Learn more
- R. Michael Akers, D. Michael Denbow(Authors)
- 2013(Publication Date)
- Wiley-Blackwell(Publisher)
Figure 3.4 . At this point, it is worth remembering the significance of Glycolysis. This pathway allows the conversion of the nutrient glucose into molecules that can then be shuttled into the mitochondria for use in the process of oxidative phosphorylation. However, as we indicated earlier, oxidative phosphorylation (simply the production of ATP linked to a series of oxidation–reduction reactions) requires oxygen. In addition to preparing molecules for entrance into the mitochondria, a small amount of ATP is produced during the Glycolysis reactions. In contrast with mitochondrial activity, this occurs via substrate-level phosphorylation. As you will see, the amount of ATP made in this manner is very small compared with that which occurs with the complete catabolism of the glucose (Glycolysis reactions + mitochondrial activity), but it is nonetheless essential. This is because production of ATP via Glycolysis alone can occur in the absence of oxygen. For this reason, it is called anaerobic respiration.Table 3.2.Common terms associated with the metabolism of carbohydrates.Term Definition Glycolysis Anaerobic oxidation of a molecule of glucose via 10 enzymatic reactions to produce two molecules of pyruvate. The reactions occur in the cytoplasm. Glycogenolysis The breakdown of glycogen to produce glucose for utilization in the Glycolysis catabolic pathway. Glycogenesis Synthesis of glycogen from glucose. Gluconeogenesis The formation of glucose from noncarbohydrate substrates. Important in times of stress, makes glucose available from nonessential amino acids. Critical in ruminants due to fermentation of dietary carbohydrates. Fig. 3.3.Chemical steps and intermediates in Glycolysis are illustrated.Fig. 3.4. - eBook - PDF
- Rodney P. Anderson, Linda Young, Kim R. Finer(Authors)
- 2020(Publication Date)
- Wiley(Publisher)
Glycolysis A series of 10 enzyme-mediated catabolic reactions that occur in the cytoplasm and degrade glucose into two mole- cules of pyruvate and generate two NADH molecules and two ATP molecules. fermentation A series of metabolic reactions that catabolize sugars into gases, acids, and other by-products while regenerating NAD + consumed during Glycolysis. 9.3 Glycolysis and Fermentation 205 6 A phosphate group binds to the third carbon of both molecules, which are then oxidized, transferring electrons to two molecules of NAD + and reducing them to two molecules of NADH. 7 Substrate-level phosphorylation transfers a phosphate group from each 1,3-bisphosphoglycerate molecule to ADP and generates two ATP. Energy input now equals energy output. 8 The phosphate groups on the third carbon of 3-phosphoglycerate are transferred to the second carbon, forming two molecules of 2-phosphoglycerate. 9 Each 2-phosphoglycerate loses a molecule of water and forms two high-energy phosphenol pyruvates. 10 Substrate-level phosphorylation generates two ATP by transferring a phosphate group from each phosphoenol pyruvate to two ADP. There is now a net energy yield of two ATP. 1,3-bisphosphoglycerate 3-phosphoglycerate 2-phosphoglycerate Phosphoenol pyruvate Pyruvate 2 2 NAD + Triose phosphate dehydrogenase Phosphoglycerate kinase ADP ATP ADP ATP O C O C C P O C O C C P O C O C C O O C O C C P P C C C P Phosphoglyceromutase Enolase Pyruvate kinase P 2 2 H 2 O NADH 1,3-bisphosphoglycerate 3-phosphoglycerate 2-phosphoglycerate Phosphoenol pyruvate Pyruvate 2 2 NAD + Triose phosphate dehydrogenase Phosphoglycerate kinase ADP ATP ADP ATP O C O C C P O C O C C P O C O C C O O C O C C P P C C C P Phosphoglyceromutase Enolase Pyruvate kinase P 2 2 H 2 O NADH Glucose ADP Glucose-6-phosphate Fructose-6-phosphate Fructose-1,6-bisphosphate Dihydroxyacetone phosphate Glyceraldehyde-3- phosphate 1 A phosphate group from ATP is transferred to the sixth carbon of glucose. - eBook - PDF
- Julianne Zedalis, John Eggebrecht(Authors)
- 2018(Publication Date)
- Openstax(Publisher)
It was probably one of the earliest metabolic pathways to evolve and is used by nearly all of the organisms on earth. Glycolysis consists of two parts: The first part prepares the six-carbon ring of glucose for cleavage into two three-carbon sugars. ATP is invested in the process during this half to energize the separation. The second half of Glycolysis extracts ATP and high-energy electrons from hydrogen atoms and attaches them to NAD + . Two ATP molecules are invested in the first half and four ATP molecules are formed by substrate phosphorylation during the second half. This produces a net gain of two ATP and two NADH molecules for the cell. 7.3 Oxidation of Pyruvate and the Citric Acid Cycle In the presence of oxygen, pyruvate is transformed into an acetyl group attached to a carrier molecule of coenzyme A. The resulting acetyl CoA can enter several pathways, but most often, the acetyl group is delivered to the citric acid cycle for further catabolism. During the conversion of pyruvate into the acetyl group, a molecule of carbon dioxide and two high- energy electrons are removed. The carbon dioxide accounts for two (conversion of two pyruvate molecules) of the six carbons of the original glucose molecule. The electrons are picked up by NAD + , and the NADH carries the electrons to a later pathway for ATP production. At this point, the glucose molecule that originally entered cellular respiration has been completely oxidized. Chemical potential energy stored within the glucose molecule has been transferred to electron carriers or has been used to synthesize a few ATPs. The citric acid cycle is a series of redox and decarboxylation reactions that remove high-energy electrons and carbon dioxide. The electrons temporarily stored in molecules of NADH and FADH 2 are used to generate ATP in a subsequent pathway. One molecule of either GTP or ATP is produced by substrate-level phosphorylation on each turn of the cycle. - eBook - PDF
- Samantha Fowler, Rebecca Roush, James Wise(Authors)
- 2016(Publication Date)
- Openstax(Publisher)
In this reaction, oxygen is consumed and carbon dioxide is released as a waste product. The reaction is summarized as: C 6 H 12 O 6 + 6O 2 -->6H 2 O + 6CO 2 Both of these reactions involve many steps. The processes of making and breaking down sugar molecules illustrate two examples of metabolic pathways. A metabolic pathway is a series of chemical reactions that takes a starting molecule and modifies it, step-by-step, through a series of metabolic intermediates, eventually yielding a final product. In the example of sugar metabolism, the first metabolic pathway synthesized sugar from smaller molecules, and the other pathway broke sugar down into smaller molecules. These two opposite processes—the first requiring energy and the second producing energy—are referred to as anabolic pathways (building polymers) and catabolic pathways (breaking down polymers into their monomers), respectively. Consequently, metabolism is composed of synthesis (anabolism) and degradation (catabolism) (Figure 4.3). It is important to know that the chemical reactions of metabolic pathways do not take place on their own. Each reaction step is facilitated, or catalyzed, by a protein called an enzyme. Enzymes are important for catalyzing all types of biological reactions—those that require energy as well as those that release energy. Figure 4.3 Catabolic pathways are those that generate energy by breaking down larger molecules. Anabolic pathways are those that require energy to synthesize larger molecules. Both types of pathways are required for maintaining the cell’s energy balance. Energy Thermodynamics refers to the study of energy and energy transfer involving physical matter. The matter relevant to a particular case of energy transfer is called a system, and everything outside of that matter is called the surroundings. For instance, when heating a pot of water on the stove, the system includes the stove, the pot, and the water. - eBook - PDF
- Donald Voet, Judith G. Voet, Charlotte W. Pratt(Authors)
- 2018(Publication Date)
- Wiley(Publisher)
Under aerobic conditions, complete oxidation of the pyruvate carbon atoms to CO 2 is mediated by the citric acid cycle (Chapter 17). The energy released in that process drives the synthesis of much more ATP than is generated by the limited oxidation of glucose by the glycolytic pathway alone. In anaerobic metabolism, pyruvate is metabolized to a lesser extent to regenerate NAD + , as we will see in the following section. 3 Fermentation: The Anaerobic Fate of Pyruvate KEY IDEAS • NADH, a substrate for the GAPDH reaction, must be reoxidized for Glycolysis to continue. • In muscle, pyruvate is reduced to lactate to regenerate NAD + . • Yeast decarboxylates pyruvate to produce CO 2 and ethanol, in a process that requires the cofactor TPP. The three common metabolic fates of pyruvate produced by Glycolysis are out- lined in Fig. 15-16. 1. Under aerobic conditions, the pyruvate is completely oxidized via the cit- ric acid cycle to CO 2 and H 2 O. 2. Under anaerobic conditions, pyruvate must be converted to a reduced end product in order to reoxidize the NADH produced by the GAPDH reaction. This occurs in two ways: (a) Under anaerobic conditions in muscle, pyruvate is reduced to lactate to regenerate NAD + in a process known as homolactic fermentation (a fermentation is an anaerobic biological process). (b) In yeast and certain other microorganisms, pyruvate is decarboxylated to yield CO 2 and acetaldehyde, which is then reduced by NADH to yield NAD + and ethanol. This process is known as alcoholic fermentation. Thus, in aerobic Glycolysis, NADH acts as a “high-energy” compound, whereas in anaerobic Glycolysis, its free energy of oxidation is dissipated as heat. REVIEW QUESTIONS 1 Write the reactions of Glycolysis, showing the structural formulas of the intermediates and the names of the enzymes that catalyze the reactions. 2 Summarize the types of catalytic mecha- nisms involved. - eBook - PDF
- Donald Voet, Judith G. Voet(Authors)
- 2023(Publication Date)
- Wiley(Publisher)
Consequently, glycogen bio- synthesis and breakdown must occur by separate pathways. Thus we encounter a recurrent metabolic strategy: Biosyn- thetic and degradative pathways of metabolism are almost always different (Section 14-1). There are two important reasons for this. The first, as we have seen, is that both pathways may be required under similar in vivo metab- olite concentrations. This situation is thermodynamically impossible if one pathway is just the reverse of the other. The second reason is equally important: Reactions cata- lyzed by different enzymes can be independently regu- lated, which permits very fine flux control. We have seen this principle in operation in the glycolytic conversion of fructose-6-phosphate (F6P) to fructose-1,6-bisphosphate (F1,6P) by phosphofructokinase (PFK; Section 15-4F). The reverse process in that case (hydrolysis of F1,6P) is catalyzed by fructose bisphosphatase (FBPase). Inde- pendent control of those two enzymes provides precise regulation of glycolytic flux. Glycogen metabolism, like Glycolysis, is exquisitely regulated by the independent control of its synthetic and degradative pathways. In the next section we examine the pathway of glycogen synthesis and, in Section 16-3, we explore the regulatory process. 2 Glycogen Synthesis Although the thermodynamic arguments presented in Section 16-1D demonstrate that glycogen synthesis and breakdown must occur by separate pathways, it was not thermodynamic arguments that led to the general accept- ance of this idea. Rather, it was the elucidation of the cause of McArdle’s disease, a rare inherited glycogen storage disease that results in painful muscle cramps on strenuous exertion (Section 16-4). The muscle tissue from individuals with McArdle’s disease exhibits no glycogen phosphoryl- ase activity and is therefore incapable of glycogen break- down. Their muscles, nevertheless, contain moderately high quantities of normal glycogen. - eBook - PDF
- Donald Voet, Charlotte W. Pratt, Judith G. Voet(Authors)
- 2014(Publication Date)
- Wiley(Publisher)
Chapter 15 Glycolysis and the Pentose Phosphate Pathway A Homolactic Fermentation Converts Pyruvate to Lactate In muscle, during vigorous activity, when the demand for ATP is high and oxygen is in short supply, ATP is largely synthesized via anaerobic Glycolysis, which rapidly generates ATP, rather than through the slower process of oxida- tive phosphorylation. Under these conditions, lactate dehydrogenase (LDH) catalyzes the oxidation of NADH by pyruvate to yield NAD and lactate. N R 4 5 3 6 1 1 2 3 2 H R H S H + + + C O – C O CH 3 O NADH NAD + Pyruvate L-Lactate N R 1 2 3 H + + C O – C O CH 3 H HO lactate dehydrogenase (LDH) C NH 2 O C NH 2 O 492 N C O H 2 N . . R C O C O O NH 2 NH 2 H 2 N H 2 N C C . . . . . . . . ... ... H – H H NH 2 NH 2 Arg 171 Arg 109 N + NH CH 2 His 195 Pyruvate NADH H + NAD + C OH O – C H O CH 3 CH 3 + + L-Lactate Both His 195 and Arg 171 interact electrostatically with the substrate to ori- ent pyruvate (or lactate, in the reverse reaction) in the enzyme active site. The overall process of anaerobic Glycolysis in muscle can be represented as Glucose 2 ADP 2 P i S 2 lactate 2 ATP 2 H 2 O 2 H Lactate represents a sort of dead end for anaerobic glucose metabolism. The lactate can either be exported from the cell or converted back to pyruvate. Much of the lactate produced in skeletal muscle cells is carried by the blood to the liver, where it is used to synthesize glucose (Section 22-1F). Contrary to widely held belief, it is not lactate buildup in the muscle per se that causes muscle fatigue and soreness but the accumulation of glycolyti- cally generated acid (muscles can maintain their workload in the presence of high lactate concentrations if the pH is kept constant). B Alcoholic Fermentation Converts Pyruvate to Ethanol and CO 2 Under anaerobic conditions in yeast, NAD for Glycolysis is regenerated in a process that has been valued for thousands of years: the conversion of pyru- vate to ethanol and CO 2 .
Index pages curate the most relevant extracts from our library of academic textbooks. They’ve been created using an in-house natural language model (NLM), each adding context and meaning to key research topics.