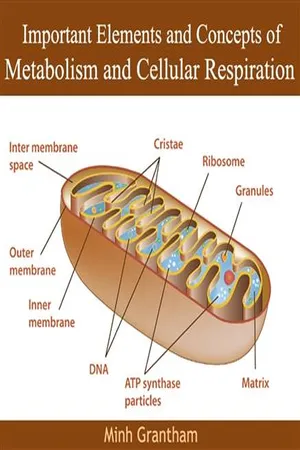
Biological Sciences
Pyruvate Oxidation
Pyruvate oxidation is a crucial step in cellular respiration where pyruvate, a product of glycolysis, is converted into acetyl-CoA. This process takes place in the mitochondria and involves the removal of a carbon dioxide molecule and the transfer of electrons to NAD+ to form NADH. The resulting acetyl-CoA then enters the citric acid cycle to further generate ATP.
Written by Perlego with AI-assistance
Related key terms
1 of 5
11 Key excerpts on "Pyruvate Oxidation"
- No longer available |Learn more
The Science of Cooking
Understanding the Biology and Chemistry Behind Food and Cooking
- Joseph J. Provost, Keri L. Colabroy, Brenda S. Kelly, Mark A. Wallert(Authors)
- 2016(Publication Date)
- Wiley(Publisher)
2 , producing the maximum amount of ATP. However if the oxygen levels fall due to exercise or other factors, anaerobic metabolism of pyruvate shifts to produce lactate. This has an important impact in meat before harvest.To review, in the presence of oxygen, pyruvate undergoes further oxidation to the molecule acetyl-CoA, which can be completely oxidized to CO2 . This process comprises aerobic respiration, which generates a large amount of ATP, H2 O, and CO2 . Under anaerobic conditions or conditions where fermentation is favored, the energy needs of the organism are met by the modest ATP yield of glycolysis as no additional ATP is generated in the further breakdown of pyruvate. In these cases, however, an organism still requires a means to regenerate NAD+ so that glycolysis can continue as the organism’s primary means to make ATP. The metabolic processes that occur to regenerate NAD+ that do not require oxygen are called fermentation processes. As we have begun to learn, fermentation is very important in cooking and foods such as yogurt, cheese, beer, wine, soy sauce, and pepperoni (Box 4.2 ).BOX 4.2 FERMENTATION VERSUS GLYCOLYSIS
Let’s consider the definition of glycolysis and fermentation. Glycolysis, as you’ve learned, is the oxidation of glucose to pyruvate. Fermentation is a broader term that includes glycolysis but includes whatever pathway is used to oxidize NAD+ back to NADH in anaerobic conditions. Thus fermentation will include glucose to pyruvate and then the continued metabolism to lactate, ethanol, acetate, carbon dioxide, or other compounds depending on the organism and conditions. Fermentation has a more loose historical definition that involves ethanol in wine and beer. Therefore fermentation is a combination of different metabolisms to produce ATP from glucose and regenerate NAD+ from NADH.4.6 AEROBIC RESPIRATION: THE TRICARBOXYLIC ACID CYCLE AND OXIDATIVE PHOSPHORYLATION
In the presence of oxygen, the pyruvate generated in glycolysis is fully oxidized to CO2 , the energy of which is used to make ATP. Three different pathways are necessary to carry out this complete oxidation. The pyruvate is first processed by a large enzyme complex called the pyruvate dehydrogenase (PDH) complex. The PDH complex, as its name implies, oxidizes and decarboxylates the pyruvate into two molecules: a two-carbon molecule called acetyl-CoA and CO2 (Fig. 4.13 ). The acetyl-CoA can then enter the second pathway, called the tricarboxylic acid (TCA) cycle, which allows for further oxidation of the molecule (Fig. 4.14 - eBook - PDF
- Rodney P. Anderson, Linda Young, Kim R. Finer(Authors)
- 2020(Publication Date)
- Wiley(Publisher)
Oxidative phosphorylation continues the degradation of glucose started in glycolysis and simultaneously regenerates the NAD + consumed. When glucose is completely oxidized to water and carbon dioxide in the presence of oxygen, its electrons reduce activated carriers that ultimately participate in the redox reactions of chemiosmosis. The end result of this metabolic pathway is typically the generation of 38 mol- ecules of ATP from a single glucose—a 19-fold increase in energy production over fermentation pathways. In eukary- otic cells, these reactions occur in the mitochondrial matrix and cristae. In prokaryotic cells, oxidative phosphorylation takes place in the cytoplasm and on infoldings of the plasma membrane. Pyruvate Oxidation and the Citric Acid Cycle The first step in this aerobic pathway is Pyruvate Oxidation. Pyru- vate dehydrogenase is a large enzyme complex that accom- plishes this and the two steps that follow (Figure 9.9). The pyruvate generated by glycolysis loses an acidic carboxyl func- tional group (COOH) and forms acetate and the by-product, carbon dioxide (step 1). During this oxidation, NAD + is reduced to NADH, storing the energy of the electrons for later use (step 2). Finally, coenzyme A (CoA) is added to acetate to form acetyl-CoA 1 Pyruvate is decarboxylated to generate acetate and carbon dioxide, which is released as a by-product. 2 Electrons from the oxidized pyruvate reduce NAD + to NADH. 3 Coenzyme A covalently binds to acetate and forms acetyl-CoA. Pyruvate Acetate 2 O C O C C O 2 O C O C Acetyl-CoA 2 2 C C O S CoA Carbon dioxide 2 Pyruvate dehydrogenase O O C NADH 2 NAD + Co A S H FIGURE 9.9 Pyruvate oxidation Using the enzyme complex pyruvate dehydrogenase, both molecules of pyruvate produced during glycolysis are oxidized in a three-step process, resulting in two molecules of NADH, two molecules of carbon dioxide, and two molecules of the substrate needed to initiate the citric acid cycle, acetyl-CoA. - eBook - PDF
- Mary Ann Clark, Jung Choi, Matthew Douglas(Authors)
- 2018(Publication Date)
- Openstax(Publisher)
The last step in glycolysis will not occur if pyruvate kinase, the enzyme that catalyzes the formation of pyruvate, is not available in sufficient quantities. In this situation, the entire glycolysis pathway will proceed, but only two ATP molecules will be made in the second half. Thus, pyruvate kinase is a rate-limiting enzyme for glycolysis. 7.3 | Oxidation of Pyruvate and the Citric Acid Cycle By the end of this section, you will be able to do the following: • Explain how a circular pathway, such as the citric acid cycle, fundamentally differs from a linear biochemical pathway, such as glycolysis • Describe how pyruvate, the product of glycolysis, is prepared for entry into the citric acid cycle If oxygen is available, aerobic respiration will go forward. In eukaryotic cells, the pyruvate molecules produced at the end of glycolysis are transported into the mitochondria, which are the sites of cellular respiration. There, pyruvate is transformed into an acetyl group that will be picked up and activated by a carrier compound called coenzyme A (CoA). The resulting compound is called acetyl CoA. CoA is derived from vitamin B5, pantothenic acid. Acetyl CoA can be used in a variety of ways by the cell, but its major function is to deliver the acetyl group derived from pyruvate to the next stage of the pathway in glucose catabolism. Breakdown of Pyruvate In order for pyruvate, the product of glycolysis, to enter the next pathway, it must undergo several changes. The conversion is a three-step process (Figure 7.8). Step 1. A carboxyl group is removed from pyruvate, releasing a molecule of carbon dioxide into the surrounding medium. This reaction creates a two-carbon hydroxyethyl group bound to the enzyme (pyruvate dehydrogenase). We should note that this is the first of the six carbons from the original glucose molecule to be removed. - eBook - PDF
- Julianne Zedalis, John Eggebrecht(Authors)
- 2018(Publication Date)
- Openstax(Publisher)
Figure 7.9 Upon entering the mitochondrial matrix, a multi-enzyme complex converts pyruvate into acetyl CoA. In the process, carbon dioxide is released and one molecule of NADH is formed. Note that during the second stage of glucose metabolism, whenever a carbon atom is removed, it is bound to two oxygen atoms, producing carbon dioxide, one of the major end products of cellular respiration. Acetyl CoA to CO 2 In the presence of oxygen, acetyl CoA delivers its acetyl group to a four-carbon molecule, oxaloacetate, to form citrate, a six-carbon molecule with three carboxyl groups; this pathway will harvest the remainder of the extractable energy from what began as a glucose molecule. This single pathway is called by different names: the citric acid cycle (for the first intermediate formed—citric acid, or citrate—when acetate joins to the oxaloacetate), the TCA cycle (since citric acid or citrate and isocitrate are tricarboxylic acids), and the Krebs cycle, after Hans Krebs, who first identified the steps in the pathway in the 1930s in pigeon flight muscles. Citric Acid Cycle Like the conversion of pyruvate to acetyl CoA, the citric acid cycle takes place in the matrix of mitochondria. Almost all of the enzymes of the citric acid cycle are soluble, with the single exception of the enzyme succinate dehydrogenase, which is embedded in the inner membrane of the mitochondrion. Unlike glycolysis, the citric acid cycle is a closed loop: The last part of the pathway regenerates the compound used in the first step. The eight steps of the cycle are a series of redox, dehydration, hydration, and decarboxylation reactions that produce two carbon dioxide molecules, one GTP/ATP, and reduced forms of NADH and FADH 2 (Figure 7.10). This is considered an aerobic pathway because the NADH and FADH 2 produced must transfer their electrons to the next pathway in the system, which will use oxygen. - eBook - PDF
- Samantha Fowler, Rebecca Roush, James Wise(Authors)
- 2016(Publication Date)
- Openstax(Publisher)
In the second part of glycolysis, ATP and nicotinamide-adenine dinucleotide (NADH) are produced (Figure 4.13). If the cell cannot catabolize the pyruvate molecules further, it will harvest only two ATP molecules from one molecule of glucose. For example, mature mammalian red blood cells are only capable of glycolysis, which is their sole source of ATP. If glycolysis is interrupted, these cells would eventually die. Chapter 4 | How Cells Obtain Energy 103 Figure 4.13 In glycolysis, a glucose molecule is converted into two pyruvate molecules. 4.3 | Citric Acid Cycle and Oxidative Phosphorylation By the end of this section, you will be able to: • Describe the location of the citric acid cycle and oxidative phosphorylation in the cell • Describe the overall outcome of the citric acid cycle and oxidative phosphorylation in terms of the products of each • Describe the relationships of glycolysis, the citric acid cycle, and oxidative phosphorylation in terms of their inputs and outputs. The Citric Acid Cycle In eukaryotic cells, the pyruvate molecules produced at the end of glycolysis are transported into mitochondria, which are sites of cellular respiration. If oxygen is available, aerobic respiration will go forward. In mitochondria, pyruvate will be transformed into a two-carbon acetyl group (by removing a molecule of carbon dioxide) that will be picked up by a carrier compound called coenzyme A (CoA), which is made from vitamin B 5 . The resulting compound is called acetyl CoA. (Figure 4.14). Acetyl CoA can be used in a variety of ways by the cell, but its major function is to deliver the acetyl group derived from pyruvate to the next pathway in glucose catabolism. 104 Chapter 4 | How Cells Obtain Energy This OpenStax book is available for free at http://cnx.org/content/col11487/1.9 Figure 4.14 Pyruvate is converted into acetyl-CoA before entering the citric acid cycle. - eBook - PDF
- Donald Voet, Judith G. Voet, Charlotte W. Pratt(Authors)
- 2018(Publication Date)
- Wiley(Publisher)
The complete oxidation of glucose (C 6 H 12 O 6 ), for example, by molecular oxygen C 6 H 12 O 6 + 6 O 2 → 6 CO 2 + 6 H 2 O can be broken down into two half-reactions that the metabolic machinery carries out. In the first, glucose carbon atoms are oxidized: C 6 H 12 O 6 + 6 H 2 O → 6 CO 2 + 24 H + + 24 e − and in the second, molecular oxygen is reduced: 6 O 2 + 24 H + + 24 e − → 12 H 2 O We have already seen that the first half-reaction is mediated by the enzymatic reactions of glycolysis and the citric acid cycle (the breakdown of fatty acids— the other major type of metabolic fuel—also requires the citric acid cycle). In this chapter, we describe the pathway by which the electrons from reduced fuel molecules are transferred to molecular oxygen in eukaryotes. We also examine how the energy of fuel oxidation is conserved and used to synthesize ATP. As we have seen, the 12 electron pairs released during glucose oxidation are not transferred directly to O 2 . Rather, they are transferred to the coenzymes NAD + and FAD to form 10 NADH and 2 FADH 2 (Fig. 18-1) in the reactions catalyzed by the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (Section 15-2F), pyruvate dehydrogenase (Section 17-2B), and the citric acid cycle enzymes isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, succinate dehydrogenase, and malate dehydrogenase (Section 17-3). The electrons then pass into the mitochondrial electron-transport chain, a system of linked electron carriers. The following events occur during the electron-transport process: 1. By transferring their electrons to other substances, the NADH and FADH 2 are reoxidized to NAD + and FAD so that they can participate in additional substrate oxidation reactions. 2. The transferred electrons participate in the sequential oxidation–reduction of multiple redox centers (groups that undergo oxidation–reduction reactions) in four enzyme complexes before reducing O 2 to H 2 O. - eBook - PDF
- Sankhavaram R. Panini(Author)
- 2013(Publication Date)
- Thieme(Publisher)
112 III Cellular Respiration 8 Principles of Cellular Respiration Cellular respiration is the process by which the energy contained within the bonds of carbohydrates, proteins, and lipids is har-vested and converted into adenosine triphosphate (ATP). The en-ergy stored in ATP is then released upon hydrolysis and used to perform mechanical work (e.g., muscle contraction) or chemical work (e.g., biomolecule synthesis). Cellular respiration depends on the integration of several metabolic pathways that link the breakdown of nutrients to oxidation-reduction (redox) reactions that culminate in the transfer of electrons to O 2 and the phos-phorylation of adenosine diphosphate (ADP) into ATP. A summary of the pathways involved follows ( Fig. 8.1 ): 1. Glycolysis: This cytosolic pathway splits glucose (a six- carbon carbohydrate) into two molecules of pyruvate (a three- carbon compound). In doing so, ATP and a reduced form of nicotinamide adenine dinucleotide (NADH) are produced. (The starting material, glucose, is obtained from the catabo-lism of carbohydrates or from de novo synthesis via gluco-neogenesis.) 2. Decarboxylation of pyruvate: Under aerobic conditions, pyruvate is transported into the mitochondria, where it is converted (via decarboxylation) to acetyl coenzyme A (CoA) in a reaction that produces NADH. Fig. 8.1 ▶ Cellular respiration In cellular respiration, four important metabolic pathways function to harvest the energy contained within the bonds of carbohydrates, proteins, and lipids and convert it to adenosine triphosphate (ATP). Those metabolic pathways are (1) glycolysis, (2) decarboxylation of pyruvate, (3) tricarboxylic acid (TCA) cycle, and (4) oxidative phosphorylation. The degradation of carbohydrates and the de novo synthesis of glucose (gluconeogenesis) supply the glucose. The degradation of lipids and proteins, as well as the metabolism of glucose, supply acetyl coenzyme A (CoA). - eBook - PDF
- P Karlson(Author)
- 2012(Publication Date)
- Academic Press(Publisher)
C H A P T E R Biological Oxidation—Metabolism of Oxygen 1. Combustion and Biological Oxidation About 1780, Lavoisier concluded that combustion processes must also take place in the animal organism. Since then, biological oxidation has often been compared to combustion. There is indeed no difference in the net equations; the end products in both cases are C 0 2 and H 2 0 , and the energy released during combustion and biological oxidation is equal, at least quantitatively. For example: Glucose C 6 H 1 2 0 6 + 6 0 2 = 6 C 0 2 + 6 H 2 0 AH = -6 7 4 kcal/mole Fatty acid C 1 6 H 3 2 0 2 + 23 0 2 = 16 C 0 2 + 16 H 2 0 AH = -2 3 7 9 kcal/mole (AH = heat of combustion at constant pressure) The analogy, however, cannot be extended much beyond this. While in technology the oxidation of carbon to C 0 2 is still the most important source of energy, the same process assumes a rather subordinate role in biochemistry. Furthermore, one characteristic feature of combustion processes is a drastic rise in temperature and unchecked evolution of heat. In the mammalian body, in contrast, all processes proceed at a constant temperature (around 37°C) and only part of the energy of oxidation appears as heat; the remainder is conserved as chemical energy. The principles of biological combustion of foodstuffs (carbohydrates, fats, and to some extent proteins) may be summarized in four sentences: 1. Complex organic molecules are first broken down to two-carbon fragments 1 (activated acetate). 2. The further breakdown of the C 2 fragments occurs in a series of separate steps, in each of which one C 0 2 or two Η atoms are split off; or, the molecule is altered so as to prepare for such a step. 1 In carbohydrates this step is reached via decarboxylation. A C 6 compound yields two C 2 fragments and two C 0 2 (Chapter XV, 9). 207 χ 208 X. BIOLOGICAL OXIDATION—METABOLISM OF OXYGEN 3. The end product C 0 2 arises by the decarboxylation of organic acids without any considerable change in energy. - eBook - PDF
- P Karlson(Author)
- 1975(Publication Date)
- Academic Press(Publisher)
C H A P T E R Biological Oxidation—Metabolism of Oxygen 1. Combustion and Biological Oxidation About 1780, Lavoisier concluded that combustion processes must also take place in the animal organism. Since then, biological oxidation has often been compared to combustion. There is indeed no difference in the net equations ; the end products in both cases are C 0 2 and H 2 0 , and the energy released during combustion and biological oxidation is equal, at least quantitatively. For example : G l u c o s e C 6 H 1 2 O e + 6 0 2 = 6 C 0 2 + 6 H 2 0 AH = -6 7 4 k c a l / m o l e Fatty acid C 1 6 H 3 2 0 2 + 23 0 2 = 16 C 0 2 + 16 H 2 0 AH = -2 3 7 9 k c a l / m o l e (AH = heat of c o m b u s t i o n at c o n s t a n t p r e s s u r e ) The analogy, however, cannot be extended much beyond this. While in technology the oxidation of carbon to C 0 2 is still the most important source of energy, the same process assumes a rather subordinate role in biochemistry. Furthermore, one characteristic feature of combustion processes is a drastic rise in temperature and unchecked evolution of heat. In the mammalian body, in contrast, all processes proceed at a constant temperature (around 37°C) and only part of the energy of oxidation appears as heat ; the remainder is conserved as chemical energy. The principles of biological combustion of foodstuffs (carbohydrates, fats, and to some extent proteins) may be summarized in four sentences : 1. Complex organic molecules are first broken down to two-carbon fragments 1 (activated acetate). 2. The further breakdown of the C 2 fragments occurs in a series of separate steps, in each of which one C 0 2 or two H atoms are split off; or, the molecule is altered so as to prepare for such a step. 1 I n carbohydrates this step is reached v i a d e c a r b o x y l a t i o n . A C 6 c o m p o u n d yields t w o C 2 fragments a n d t w o C 0 2 ( C h a p t e r X V , 9). 2 0 7 - No longer available |Learn more
- (Author)
- 2014(Publication Date)
- University Publications(Publisher)
• Third, the NADH is oxidized to NAD + by the electron transport chain, using oxygen as the final electron acceptor. This process creates a hydrogen ion gradient across the inner membrane of the mitochondria. • Fourth, the proton gradient is used to produce a large amount of ATP in a process called oxidative phosphorylation. Intermediates for other pathways ________________________ WORLD TECHNOLOGIES ________________________ Here we, concentrates on the catabolic role of glycolysis with regard to converting potential chemical energy to usable chemical energy during the oxidation of glucose to pyruvate. However, many of the metabolites in the glycolytic pathway are also used by anabolic pathways, and, as a consequence, flux through the pathway is critical to maintain a supply of carbon skeletons for biosynthesis. In addition, not all carbon entering the pathway leaves as pyruvate and may be extracted at earlier stages to provide carbon compounds for other pathways. These metabolic pathways are all strongly reliant on glycolysis as a source of metabolites: • Gluconeogenesis • Lipid metabolism • Pentose phosphate pathway • Citric acid cycle, which in turn leads to: • Amino acid synthesis • Nucleotide synthesis • Tetrapyrrole synthesis From an anabolic metabolism perspective, the NADH has a role to drive synthetic reactions, doing so by directly or indirectly reducing the pool of NADP+ in the cell to NADPH, which is another important reducing agent for biosynthetic pathways in a cell. Glycolysis in disease Genetic diseases Glycolytic mutations are generally rare due to importance of the metabolic pathway, this means that the majority of occurring mutations result in an inability for the cell to respire, and therefore cause the death of the cell at an early stage. However, some mutations are seen with one notable example being Pyruvate kinase deficiency, leading to chronic hemolytic anemia. - eBook - PDF
- Charlotte W. Pratt, Kathleen Cornely(Authors)
- 2020(Publication Date)
- Wiley(Publisher)
2. Like ATP, carboxyphosphate releases a large amount of free energy when its phosphoryl group is liberated. This free energy drives the carboxylation of biotin. 4. The carbanion attacks the carboxyl group attached to biotin, generating oxaloacetate. 3. The enzyme abstracts a proton from pyruvate, forming a carbanion. FIGURE 13.9 The pyruvate carboxylase reaction. KEY CONCEPTS • Pyruvate is converted to glucose by glycolytic enzymes operating in reverse and by enzymes that bypass the irreversible steps of glycolysis. • Gluconeogenic flux is regulated primarily by fructose-2,6-bisphosphate. We have already alluded to the ability of the liver to synthesize glucose from non- carbohydrate precursors via the pathway of gluconeogenesis. This pathway, which also occurs to a limited extent in the kidneys, operates when the liver’s supply of glycogen is exhausted. Certain tissues, such as the central nervous system and red blood cells, which burn glucose as their primary metabolic fuel, rely on the liver to supply them with newly synthesized glucose. Gluconeogenesis is considered to be the reversal of glycolysis, that is, the conversion of two molecules of pyruvate to one molecule of glucose. Although some of the steps of gluco- neogenesis are catalyzed by glycolytic enzymes operating in reverse, the gluconeogenic path- way contains several unique enzymes that bypass the three irreversible steps of glycolysis— the steps catalyzed by pyruvate kinase, phosphofructokinase, and hexokinase (Fig. 13.10). This principle applies to all pairs of opposing metabolic pathways: The pathways may share some near-equilibrium reactions but cannot use the same enzymes to catalyze thermodynam- ically favorable irreversible reactions. The three irreversible reactions of glycolysis are clearly visible in the “waterfall” diagram (see Fig. 13.7). GLUCONEOGENESIS 13.2
Index pages curate the most relevant extracts from our library of academic textbooks. They’ve been created using an in-house natural language model (NLM), each adding context and meaning to key research topics.